Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms
- PMID: 18703050
- PMCID: PMC2570357
- DOI: 10.1016/j.yexcr.2008.07.021
Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms
Abstract
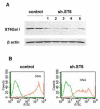
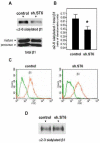
The ST6Gal-I glycosyltransferase, which adds alpha2-6-linked sialic acids to glycoproteins, is overexpressed in colon adenocarcinoma, and enzyme activity is correlated with tumor cell invasiveness. Previously we reported that forced expression of oncogenic ras in HD3 colonocytes causes upregulation of ST6Gal-I, leading to increased alpha2-6 sialylation of beta1 integrins. To determine whether ras-induced sialylation is involved in promoting the tumor cell phenotype, we used shRNA to downregulate ST6Gal-I in ras-expressors, and then monitored integrin-dependent responses. Here we show that forced ST6Gal-I downregulation, leading to diminished alpha2-6 sialylation of integrins, inhibits cell adhesion to collagen I, a beta1 ligand. Correspondingly, collagen binding is reduced by enzymatic removal of cell surface sialic acids from ras-expressors with high ST6Gal-I levels (i.e., no shRNA). Cells with forced ST6Gal-I downregulation also exhibit decreased migration on collagen I and diminished invasion through Matrigel. Importantly, GD25 cells, which lack beta1 integrins (and ST6Gal-I), do not demonstrate differential invasiveness when forced to express ST6Gal-I, suggesting that the effects of variant sialylation are mediated specifically by beta1 integrins. The observation that cell migration and invasion can be blocked in oncogenic ras-expressing cells by forcing ST6Gal-I downregulation implicates differential sialylation as an important ras effector, and also suggests that ST6Gal-I is a promising therapeutic target.
Figures



Similar articles
-
Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility.Cancer Res. 2005 Jun 1;65(11):4645-52. doi: 10.1158/0008-5472.CAN-04-3117. Cancer Res. 2005. PMID: 15930282
-
Cleavage of ST6Gal I by radiation-induced BACE1 inhibits golgi-anchored ST6Gal I-mediated sialylation of integrin β1 and migration in colon cancer cells.Radiat Oncol. 2012 Mar 27;7:47. doi: 10.1186/1748-717X-7-47. Radiat Oncol. 2012. PMID: 22449099 Free PMC article.
-
Ras oncogene directs expression of a differentially sialylated, functionally altered beta1 integrin.Oncogene. 2003 Oct 16;22(46):7137-45. doi: 10.1038/sj.onc.1206834. Oncogene. 2003. PMID: 14562042
-
Significance of β-Galactoside α2,6 Sialyltranferase 1 in Cancers.Molecules. 2015 Apr 24;20(5):7509-27. doi: 10.3390/molecules20057509. Molecules. 2015. PMID: 25919275 Free PMC article. Review.
-
The sialyl-alpha2,6-lactosaminyl-structure: biosynthesis and functional role.Glycoconj J. 2000 Oct;17(10):669-76. doi: 10.1023/a:1011077000164. Glycoconj J. 2000. PMID: 11425186 Review.
Cited by
-
ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells.J Ovarian Res. 2013 Apr 11;6(1):25. doi: 10.1186/1757-2215-6-25. J Ovarian Res. 2013. PMID: 23578204 Free PMC article.
-
Stable Ectopic Expression of ST6GALNAC5 Induces Autocrine MET Activation and Anchorage-Independence in MDCK Cells.PLoS One. 2016 Feb 5;11(2):e0148075. doi: 10.1371/journal.pone.0148075. eCollection 2016. PLoS One. 2016. PMID: 26848584 Free PMC article.
-
Regulation of the metastatic cell phenotype by sialylated glycans.Cancer Metastasis Rev. 2012 Dec;31(3-4):501-18. doi: 10.1007/s10555-012-9359-7. Cancer Metastasis Rev. 2012. PMID: 22699311 Free PMC article. Review.
-
Transfer of Functional Cargo in Exomeres.Cell Rep. 2019 Apr 16;27(3):940-954.e6. doi: 10.1016/j.celrep.2019.01.009. Epub 2019 Apr 4. Cell Rep. 2019. PMID: 30956133 Free PMC article.
-
Clinicopathological utility of sialoglycoconjugates in diagnosing and treating colorectal cancer.World J Gastroenterol. 2014 May 28;20(20):6123-32. doi: 10.3748/wjg.v20.i20.6123. World J Gastroenterol. 2014. PMID: 24876734 Free PMC article. Review.
References
-
- Dall'Olio F, Chiricolo M. Sialyltransferases in cancer. Glycoconj J. 2001;18:841–850. - PubMed
-
- Dall'Olio F. The sialyl-alpha2,6-lactosaminyl-structure: biosynthesis and functional role. Glycoconj J. 2000;17:669–676. - PubMed
-
- Dall'Olio F, Chiricolo M, Ceccarelli C, Minni F, Marrano D, Santini D. Beta-galactoside alpha2,6 sialyltransferase in human colon cancer: contribution of multiple transcripts to regulation of enzyme activity and reactivity with Sambucus nigra agglutinin. Int J Cancer. 2000;88:58–65. - PubMed
-
- Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J. 1997;14:569–576. - PubMed
-
- Dennis JW, Granovsky M, Warren CE. Protein glycosylation in development and disease. Bioessays. 1999;21:412–421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

