Maintaining epitheliopoietic potency when culturing olfactory progenitors
- PMID: 18703052
- PMCID: PMC2888843
- DOI: 10.1016/j.expneurol.2008.07.012
Maintaining epitheliopoietic potency when culturing olfactory progenitors
Abstract
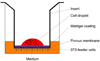
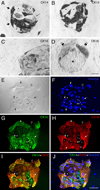
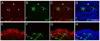
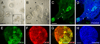
The olfactory epithelium is remarkable for the persistence of multipotent, neurocompetent progenitor and stem cells throughout life that can replace all of the various cell types of the epithelium following injury. The therapeutic exploitation of the neurocompetent stem cells of the adult olfactory epithelium would be facilitated by the development of a culture system that maintains the in vivo potency of the progenitors while they are expanded and/or manipulated. We have used an air-liquid interface culture protocol, in which a feeder cell layer of 3T3 cells is established on the underside of a culture insert and Facs-isolated or unsorted progenitor cells from the methyl bromide-lesioned adult rodent epithelium are seeded on upper side. Under these conditions, epithelial cells other than HBCs are capable of organizing themselves into complex three-dimensional, epithelium-lined spheres, which can be passaged. The spheres contain cells with the molecular phenotype of globose basal cells, horizontal basal cells, sustentacular cells and neurons. Spheres derived from mice that express the green fluorescent protein constitutively can be dissociated after 6 days in vitro and directly transplanted into the epithelium of wild-type, methyl bromide-lesioned mice via nasal infusion. The resulting clones contain the various cell types observed in aggregate when globose basal cells are transplanted acutely. In contrast, the same cells cultured as two-dimensional, submerged cultures undergo fibroblastic transition after transplantation and do not integrate into the epithelium. In conclusion, the culture system described here maintains the potency of progenitors, which can then participate in epitheliopoiesis in vivo.
Figures





Similar articles
-
Multipotency of purified, transplanted globose basal cells in olfactory epithelium.J Comp Neurol. 2004 Feb 16;469(4):457-74. doi: 10.1002/cne.11031. J Comp Neurol. 2004. PMID: 14755529
-
The generation of olfactory epithelial neurospheres in vitro predicts engraftment capacity following transplantation in vivo.Exp Neurol. 2011 Jun;229(2):308-23. doi: 10.1016/j.expneurol.2011.02.014. Epub 2011 Mar 1. Exp Neurol. 2011. PMID: 21376038 Free PMC article.
-
Expression of pax6 and sox2 in adult olfactory epithelium.J Comp Neurol. 2010 Nov 1;518(21):4395-418. doi: 10.1002/cne.22463. J Comp Neurol. 2010. PMID: 20852734 Free PMC article.
-
Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license.J Comp Neurol. 2017 Mar 1;525(4):1034-1054. doi: 10.1002/cne.24105. Epub 2016 Sep 27. J Comp Neurol. 2017. PMID: 27560601 Free PMC article. Review.
-
Neural regeneration and the peripheral olfactory system.Anat Rec. 2002 Feb 15;269(1):33-49. doi: 10.1002/ar.10047. Anat Rec. 2002. PMID: 11891623 Review.
Cited by
-
Translational potential of olfactory mucosa for the study of neuropsychiatric illness.Transl Psychiatry. 2015 Mar 17;5(3):e527. doi: 10.1038/tp.2014.141. Transl Psychiatry. 2015. PMID: 25781226 Free PMC article. Review.
-
Quiescent horizontal basal stem cells act as a niche for olfactory neurogenesis in a mouse 3D organoid model.Cell Rep Methods. 2025 Jun 16;5(6):101055. doi: 10.1016/j.crmeth.2025.101055. Epub 2025 May 28. Cell Rep Methods. 2025. PMID: 40441150 Free PMC article.
-
Molecular events in the cell types of the olfactory epithelium during adult neurogenesis.Mol Brain. 2013 Nov 22;6:49. doi: 10.1186/1756-6606-6-49. Mol Brain. 2013. PMID: 24267470 Free PMC article.
-
Pathophysiology of Olfactory Disorders and Potential Treatment Strategies.Curr Otorhinolaryngol Rep. 2016 Jun;4(2):115-121. doi: 10.1007/s40136-016-0113-5. Curr Otorhinolaryngol Rep. 2016. PMID: 27529054 Free PMC article.
-
Differentiation potential of individual olfactory c-Kit+ progenitors determined via multicolor lineage tracing.Dev Neurobiol. 2016 Mar;76(3):241-51. doi: 10.1002/dneu.22310. Epub 2015 Jun 10. Dev Neurobiol. 2016. PMID: 26016700 Free PMC article.
References
-
- Asselineau D, Bernhard B, Bailly C, Darmon M. Epidermal morphogenesis and induction of the 67 kD keratin polypeptide by culture of human keratinocytes at the liquid-air interface. Exp Cell Res. 1985;159:536–539. - PubMed
-
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. - PubMed
-
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. - PubMed
-
- Cau E, Gradwohl G, Fode C, Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

