Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors
- PMID: 18703061
- PMCID: PMC2612755
- DOI: 10.1053/j.gastro.2008.05.047
Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors
Abstract
Pancreatic endocrine tumors (PETs) have long fascinated clinicians and investigators despite their relative rarity. Their clinical presentation varies depending on whether the tumor is functional or not, and also according to the specific hormonal syndrome produced. Tumors may be sporadic or inherited, but little is known about their molecular pathology, especially the sporadic forms. Chromogranin A appears to be the most useful serum marker for diagnosis, staging, and monitoring. Initially, therapy should be directed at the hormonal syndrome because this has the major initial impact on the patient's health. Most PETs are relatively indolent but ultimately malignant, except for insulinomas, which predominantly are benign. Surgery is the only modality that offers the possibility of cure, although it generally is noncurative in patients with Zollinger-Ellison syndrome or nonfunctional PETs with multiple endocrine neoplasia-type 1. Preoperative staging of disease extent is necessary to determine the likelihood of complete resection although debulking surgery often is believed to be useful in patients with unresectable tumors. Once metastatic, biotherapy is usually the first modality used because it generally is well tolerated. Systemic or regional therapies generally are reserved until symptoms occur or tumor growth is rapid. Recently, a number of newer agents, as well as receptor-directed radiotherapy, are being evaluated for patients with advanced disease. This review addresses a number of recent advances regarding the molecular pathology, diagnosis, localization, and management of PETs including discussion of peptide-receptor radionuclide therapy and other novel antitumor approaches. We conclude with a discussion of future directions and unsettled problems in the field.
Figures

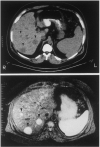

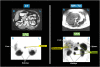

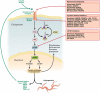
References
-
- Duerr EM, Chung DC. Molecular genetics of neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab. 2007;21:1–14. - PubMed
-
- Corleto VD, Delle Fave G, Jensen RT. Molecular insights into gastrointestinal neuroendocrine tumors: importance and recent advances. Dig Liver Dis. 2002;34:668–680. - PubMed
-
- Arnold R, editor. Endocrine Tumors of the Gastrointestinal Tract: Part 11. 2005.
-
- Arnold R, editor. Endocrine tumors of the Gastrointestinal Tract: Part 1. 2005.
-
- Oberg K, Eriksson BE. Neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab. 2007;21:1–172. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

