Evaluation of the tolerogenic effects of donor bone marrow cells using a severe combined immunodeficient mouse-human islet transplant model
- PMID: 18703102
- PMCID: PMC4094136
- DOI: 10.1016/j.humimm.2008.07.003
Evaluation of the tolerogenic effects of donor bone marrow cells using a severe combined immunodeficient mouse-human islet transplant model
Abstract
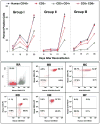
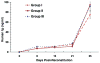
The immunoregulatory role of human donor bone marrow cells (DBMC) has been studied extensively in our laboratory using in vitro and ex vivo assays. However, new experimental systems that can overcome the limitations of tissue culture assays but with more clinical relevance than purely animal experimentation, needed to be generated. Therefore we have developed a new human peripheral blood lymphocyte (PBL) severe combined immunodeficient (SCID) mouse islet transplantation model without the occurrence of graft-versus-host disease (GvHD) and have used it to evaluate the tolerogenic effects of DBMC. Nonobese diabetogenic (NOD)-SCID mice were transplanted with human deceased donor islets and were reconstituted with human PBL (allogeneic to islets; denoted as recipient) with or without DBMC from the islet donor. It was observed that the most cellularly economical dose was 3000 islets per animal and that injection into the portal vein was better than implantation under the kidney capsule. Even though maximal lymphoid reconstitution was observed with 40-million fresh and anti-CD3 activated recipient PBL (conventional method), the mice developed severe graft GvHD. However, with the new method of reconstitution where animals were injected with 20-million anti-CD3-activated plus 40-million anti-donor-activated recipient PBL, no discernible GvHD was observed. More importantly, this latter method was associated with islet transplant rejection, which in turn could be abrogated by co-injection of the animals with DBMC. These in vivo results confirmed our previous in vitro observations that human DBMC have regulatory activity.
Figures



Similar articles
-
Continuing observations on the regulatory effects of donor-specific bone marrow cell infusions and chimerism in kidney transplant recipients.Transplantation. 1998 Apr 15;65(7):956-65. doi: 10.1097/00007890-199804150-00016. Transplantation. 1998. PMID: 9565101
-
Human peripheral blood lymphocyte reconstituted severe combined immunodeficient (hu-PBL-SCID) mice. A model for human islet allograft rejection.Transplantation. 1994 Jun 15;57(11):1555-62. Transplantation. 1994. PMID: 8009588
-
Rejection of human islets and human HLA-A2.1 transgenic mouse islets by alloreactive human lymphocytes in immunodeficient NOD-scid and NOD-Rag1(null)Prf1(null) mice.Clin Immunol. 2004 Sep;112(3):273-83. doi: 10.1016/j.clim.2004.04.006. Clin Immunol. 2004. PMID: 15308121
-
Immune responses and their regulation by donor bone marrow cells in clinical organ transplantation.Transpl Immunol. 2003 Jul-Sep;11(3-4):307-21. doi: 10.1016/S0966-3274(03)00056-X. Transpl Immunol. 2003. PMID: 12967784 Review.
-
Donor bone marrow infusion in deceased and living donor renal transplantation.Yonsei Med J. 2004 Dec 31;45(6):998-1003. doi: 10.3349/ymj.2004.45.6.998. Yonsei Med J. 2004. PMID: 15627290 Review.
Cited by
-
Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngeneic mouse model.Diabetologia. 2014 Mar;57(3):522-31. doi: 10.1007/s00125-013-3109-4. Epub 2013 Nov 20. Diabetologia. 2014. PMID: 24253203
-
Human allograft rejection in humanized mice: a historical perspective.Cell Mol Immunol. 2012 May;9(3):225-31. doi: 10.1038/cmi.2011.64. Epub 2012 Feb 13. Cell Mol Immunol. 2012. PMID: 22327213 Free PMC article. Review.
References
-
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603. - PubMed
-
- Barber WH, Mankin JA, Laskow DA, Deierhoi MH, Julian BA, Curtis JJ, Diethelm AG. Long-term results of a controlled prospective study with transfusion of donor-specific bone marrow in 57 cadaveric renal allograft recipients. Transplantation. 1991;51:70. - PubMed
-
- Caridis DT, Liegeois A, Barrett I, Monaco AP. Enhanced survival of canine renal allografts of ALS-treated dogs given bone marrow. Transplant Proc. 1973;5:671. - PubMed
-
- Monaco AP, Clark AW, Wood ML, Sahyoun AI, Codish SD, Brown RW. Possible active enhancement of a human cadaver renal allograft with antilymphocyte serum (ALS) and donor bone marrow: Case report of an initial attempt. Surgery. 1976;79:384. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

