Dissection and identification of regions required to form pseudoparticles by the interaction between the nucleocapsid (N) and membrane (M) proteins of SARS coronavirus
- PMID: 18703211
- PMCID: PMC7103410
- DOI: 10.1016/j.virol.2008.07.012
Dissection and identification of regions required to form pseudoparticles by the interaction between the nucleocapsid (N) and membrane (M) proteins of SARS coronavirus
Abstract
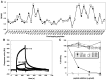
When expressed in mammalian cells, the nucleocapsid (N) and membrane (M) proteins of the severe acute respiratory syndrome coronavirus (SARS-CoV) are sufficient to form pseudoparticles. To identify region(s) of the N molecule required for pseudoparticle formation, we performed biochemical analysis of the interaction of N mutants and M in HEK293 cells. Using a peptide library derived from N, we found that amino acids 101-115 constituted a novel binding site for M. We examined the ability of N mutants to interact with M and form pseudoparticles, and our observations indicated that M bound to NDelta(101-115), N1-150, N151-300, and N301-422, but not to N1-150Delta(101-115). However, pseudoparticles were formed when NDelta(101-115) or N301-422, but not N1-150 or N151-300, were expressed with M in HEK293 cells. These results indicated that the minimum portion of N required for the interaction with M and pseudoparticle formation consists of amino acids 301-422.
Figures




Similar articles
-
Carboxyl terminus of severe acute respiratory syndrome coronavirus nucleocapsid protein: self-association analysis and nucleic acid binding characterization.Biochemistry. 2006 Oct 3;45(39):11827-35. doi: 10.1021/bi0609319. Biochemistry. 2006. PMID: 17002283
-
Characterization of protein-protein interactions between the nucleocapsid protein and membrane protein of the SARS coronavirus.Virus Res. 2004 Oct;105(2):121-5. doi: 10.1016/j.virusres.2004.05.002. Virus Res. 2004. PMID: 15351485 Free PMC article.
-
Self-assembly of severe acute respiratory syndrome coronavirus membrane protein.J Biol Chem. 2010 Apr 23;285(17):12862-72. doi: 10.1074/jbc.M109.030270. Epub 2010 Feb 12. J Biol Chem. 2010. PMID: 20154085 Free PMC article.
-
The SARS coronavirus nucleocapsid protein--forms and functions.Antiviral Res. 2014 Mar;103:39-50. doi: 10.1016/j.antiviral.2013.12.009. Epub 2014 Jan 11. Antiviral Res. 2014. PMID: 24418573 Free PMC article. Review.
-
An overall picture of SARS coronavirus (SARS-CoV) genome-encoded major proteins: structures, functions and drug development.Curr Pharm Des. 2006;12(35):4539-53. doi: 10.2174/138161206779010459. Curr Pharm Des. 2006. PMID: 17168760 Review.
Cited by
-
Druggable targets from coronaviruses for designing new antiviral drugs.Bioorg Med Chem. 2020 Nov 15;28(22):115745. doi: 10.1016/j.bmc.2020.115745. Epub 2020 Sep 8. Bioorg Med Chem. 2020. PMID: 33007557 Free PMC article. Review.
-
Viroporins: Structure, function, and their role in the life cycle of SARS-CoV-2.Int J Biochem Cell Biol. 2022 Apr;145:106185. doi: 10.1016/j.biocel.2022.106185. Epub 2022 Feb 24. Int J Biochem Cell Biol. 2022. PMID: 35219876 Free PMC article. Review.
-
Development of an immunochromatographic assay specifically detecting pandemic H1N1 (2009) influenza virus.J Clin Microbiol. 2010 Mar;48(3):703-8. doi: 10.1128/JCM.02262-09. Epub 2010 Jan 13. J Clin Microbiol. 2010. PMID: 20071549 Free PMC article.
-
Natural Bioactive Molecules as Potential Agents Against SARS-CoV-2.Front Pharmacol. 2021 Aug 17;12:702472. doi: 10.3389/fphar.2021.702472. eCollection 2021. Front Pharmacol. 2021. PMID: 34483904 Free PMC article. Review.
-
Identifying SARS-CoV membrane protein amino acid residues linked to virus-like particle assembly.PLoS One. 2013 May 20;8(5):e64013. doi: 10.1371/journal.pone.0064013. Print 2013. PLoS One. 2013. PMID: 23700447 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

