Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis
- PMID: 18703335
- PMCID: PMC2628975
- DOI: 10.1016/j.tcb.2008.07.003
Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis
Abstract
Cells use a variety of intercellular structures, including gap junctions and synapses, for cell-cell communication. Here, we present recent advances in the understanding of thin membrane bridges that function in cell-cell signaling and intercellular transport. Cytonemes or filopodial bridges connect neighboring cells via mechanisms of adhesion, which enable ligand-receptor-mediated transfer of surface-associated cargoes from cell to cell. By contrast, tunneling nanotubes establish tubular conduits between cells that provide for the exchange of both cell-surface molecules and cytoplasmic content. We propose models for the biogenesis of both types of membrane bridges and describe how viruses use these structures for the purpose of cell-to-cell spread.
Figures
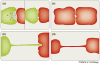


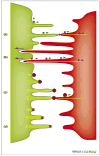
References
-
- Ramirez-Weber FA, Kornberg TB. Signaling reaches to new dimensions in Drosophila imaginal discs. Cell. 2000;103:189–192. - PubMed
-
- Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–436. - PubMed
-
- Gerdes HH, Carvalho R. Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20:470–475. - PubMed
-
- Rustom A, et al. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

