Association of the protein D and protein E forms of rat CRISP1 with epididymal sperm
- PMID: 18703418
- PMCID: PMC2844498
- DOI: 10.1095/biolreprod.108.070664
Association of the protein D and protein E forms of rat CRISP1 with epididymal sperm
Abstract
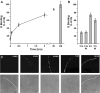
Cysteine-rich secretory protein 1 (CRISP1) is a secretory glycoprotein produced by the rat epididymal epithelium in two forms, referred to as proteins D and E. CRISP1 has been implicated in sperm-egg fusion and has been shown to suppress capacitation in rat sperm. Several studies have suggested that CRISP1 associates transiently with the sperm surface, whereas others have shown that at least a portion of CRISP1 persists on the surface. In the present study, we demonstrate that protein D associates transiently with the sperm surface in a concentration-dependent manner, exhibiting saturable binding to both caput and cauda sperm in a concentration range that is consistent with its capacitation-inhibiting activity. In contrast, protein E persists on the sperm surface after all exogenous protein D has been dissociated. Comparison of caput and cauda sperm reveal that protein E becomes bound to the sperm in the cauda epididymidis. We show that protein E associates with caput sperm, which do not normally have it on their surfaces, in vitro in a time- and temperature-dependent manner. These studies demonstrate that most CRISP1 interacts with sperm transiently, possibly with a specific receptor on the sperm surface, consistent with its action in suppressing capacitation during epididymal storage of sperm. These studies also confirm a tightly bound population of protein E that could act in the female tract.
Figures






References
-
- Gibbs GM, Scanlon MJ, Swarbrick J, Curtis S, Gallant E, Dulhunty AF, O'Bryan MK.The cysteine-rich secretory protein domain of Tpx-1 is related to ion channel toxins and regulates ryanodine receptor Ca2+ signaling. J Biol Chem 2006; 281: 4156–4163. - PubMed
-
- Guo M, Teng M, Niu L, Liu Q, Huang Q, Hao Q.Crystal structure of the cysteine-rich secretory protein stecrisp reveals that the cysteine-rich domain has a K+ channel inhibitor-like fold. J Biol Chem 2005; 280: 12405–12412. - PubMed
-
- Shikamoto Y, Suto K, Yamazaki Y, Morita T, Mizuno H.Crystal structure of a CRISP family Ca2+ -channel blocker derived from snake venom. J Mol Biol 2005; 350: 735–743. - PubMed
-
- Wang F, Li H, Liu MN, Song H, Han HM, Wang QL, Yin CC, Zhou YC, Qi Z, Shu YY, Lin ZJ, Jiang T.Structural and functional analysis of natrin, a venom protein that targets various ion channels. Biochem Biophys Res Commun 2006; 351: 443–448. - PubMed
-
- Wang J, Shen B, Guo M, Lou X, Duan Y, Cheng XP, Teng M, Niu L, Liu Q, Huang Q, Hao Q.Blocking effect and crystal structure of natrin toxin, a cysteine-rich secretory protein from Naja atra venom that targets the BKCa channel. Biochemistry 2005; 44: 10145–10152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

