UBE2I (UBC9), a SUMO-conjugating enzyme, localizes to nuclear speckles and stimulates transcription in mouse oocytes
- PMID: 18703419
- PMCID: PMC2714998
- DOI: 10.1095/biolreprod.108.070474
UBE2I (UBC9), a SUMO-conjugating enzyme, localizes to nuclear speckles and stimulates transcription in mouse oocytes
Abstract
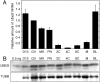
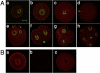
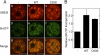
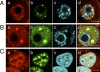
Sumoylation is a posttranslational modification in which SUMO (small ubiquitin-related modifier) proteins are covalently attached to their substrates. In vertebrates, developmental roles for sumoylation have been studied, but the function of sumoylation during mammalian oocyte growth and maturation is not known. As a prelude to conducting studies on the role of sumoylation during oocyte development, we analyzed the temporal and spatial pattern of expression of UBE2I, a SUMO-conjugating E2 enzyme. Immunocytochemical analysis of UBE2I revealed a punctate nuclear staining pattern, with transcriptionally quiescent, fully grown, GV-intact oocytes having larger UBE2I-containing bodies than transcriptionally active, meiotically incompetent growing oocytes. Inhibiting transcription in incompetent oocytes resulted in an increase in the size of the UBE2I-containing bodies. Overexpression of either wild-type UBE2I or catalytically inactive UBE2I resulted in an increase in the size of the UBE2I-containing bodies but also an increase in BrUTP incorporation, suggesting that transcriptional activation by UBE2I is independent of its catalytic activity. Although UBE2I-containing bodies did not completely colocalize with SUMO1 or SUMO2 and SUMO3, which were localized mainly on the nuclear membrane and in the nucleoplasm, UBE2I strikingly colocalized with SFRS2, which is a component of nuclear speckles and critical for mRNA processing. These results suggest a novel function for UBE2I and therefore sumoylation in gene expression.
Figures


References
-
- Johnson E.Protein modification by SUMO. Annu Rev Biochem 2004; 73: 355–382.. - PubMed
-
- Geiss-Friedlander R, Melchior F.Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 2007; 8: 947–956.. - PubMed
-
- Hayashi T, Seki M, Maeda D, Wang W, Kawabe Y, Seki T, Saitoh H, Fukagawa T, Yagi H, Enomoto T.Ubc9 is essential for viability of higher eukaryotic cells. Exp Cell Res 2002; 280: 212–221.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

