Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria
- PMID: 18703498
- PMCID: PMC2566526
- DOI: 10.1093/hmg/ddn226
Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria
Abstract
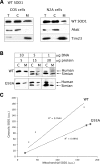
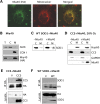
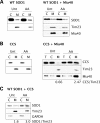
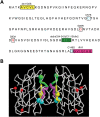
The antioxidant enzyme Cu,Zn superoxide dismutase (SOD1) is predominantly localized in the cytosol, but it is also found in mitochondria. Studies in yeast suggest that apoSOD1 is imported into mitochondria and trapped inside by folding and maturation, which is facilitated by its copper chaperone for SOD1 (CCS). Here, we show that in mammalian cells, SOD1 mitochondrial localization is dictated by its folding state, which is modulated by several interconnected factors. First, the intracellular distribution of CCS determines SOD1 partitioning in cytosol and mitochondria: CCS localization in the cytosol prevents SOD1 mitochondrial import, whereas CCS in mitochondria increases it. Second, the Mia40/Erv1 pathway for import of small intermembrane space proteins participates in CCS mitochondrial import in a respiratory chain-dependent manner. Third, CCS mitochondrial import is regulated by oxygen concentration: high (20%) oxygen prevents import, whereas physiological (6%) oxygen promotes it. Therefore, SOD1 localization responds to changes in environmental conditions following redistribution of CCS, which operates as an oxygen sensor. Fourth, all of the cysteine residues in human SOD1 are critical for its retention in mitochondria due to their involvement in intramolecular disulfide bonds and in the interaction with CCS. Mutations in SOD1 are associated with autosomal dominant familial amyotrophic lateral sclerosis. Like the wild-type protein, mutant SOD1 localizes to mitochondria, where it induces bioenergetic defects. We find that the physiological regulation of mitochondrial localization is either inefficient or absent in SOD1 pathogenic mutants. We propose misfolding and aggregation of these mutants that trap them inside mitochondria.
Figures



Similar articles
-
Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space.Antioxid Redox Signal. 2010 Nov 1;13(9):1375-84. doi: 10.1089/ars.2010.3212. Antioxid Redox Signal. 2010. PMID: 20367259 Free PMC article. Review.
-
Mia40 and MINOS act in parallel with Ccs1 in the biogenesis of mitochondrial Sod1.FEBS J. 2013 Oct;280(20):4943-59. doi: 10.1111/febs.12409. Epub 2013 Jul 22. FEBS J. 2013. PMID: 23802566
-
Mia40-dependent oxidation of cysteines in domain I of Ccs1 controls its distribution between mitochondria and the cytosol.Mol Biol Cell. 2011 Oct;22(20):3749-57. doi: 10.1091/mbc.E11-04-0293. Epub 2011 Aug 24. Mol Biol Cell. 2011. PMID: 21865594 Free PMC article.
-
The disulfide relay system of mitochondria is required for the biogenesis of mitochondrial Ccs1 and Sod1.J Mol Biol. 2009 Jan 16;385(2):331-8. doi: 10.1016/j.jmb.2008.10.088. Epub 2008 Nov 7. J Mol Biol. 2009. PMID: 19010334
-
Posttranslational modifications in Cu,Zn-superoxide dismutase and mutations associated with amyotrophic lateral sclerosis.Antioxid Redox Signal. 2006 May-Jun;8(5-6):847-67. doi: 10.1089/ars.2006.8.847. Antioxid Redox Signal. 2006. PMID: 16771675 Free PMC article. Review.
Cited by
-
Palmitoylation of superoxide dismutase 1 (SOD1) is increased for familial amyotrophic lateral sclerosis-linked SOD1 mutants.J Biol Chem. 2013 Jul 26;288(30):21606-17. doi: 10.1074/jbc.M113.487231. Epub 2013 Jun 12. J Biol Chem. 2013. PMID: 23760509 Free PMC article.
-
Redox Mechanisms in Neurodegeneration: From Disease Outcomes to Therapeutic Opportunities.Antioxid Redox Signal. 2019 Apr 10;30(11):1450-1499. doi: 10.1089/ars.2017.7321. Epub 2018 May 4. Antioxid Redox Signal. 2019. PMID: 29634350 Free PMC article. Review.
-
Redox properties of the disulfide bond of human Cu,Zn superoxide dismutase and the effects of human glutaredoxin 1.Biochem J. 2012 Aug 15;446(1):59-67. doi: 10.1042/BJ20120075. Biochem J. 2012. PMID: 22651090 Free PMC article.
-
Oxidative Stress in Amyotrophic Lateral Sclerosis: Synergy of Genetic and Environmental Factors.Int J Mol Sci. 2022 Aug 19;23(16):9339. doi: 10.3390/ijms23169339. Int J Mol Sci. 2022. PMID: 36012603 Free PMC article. Review.
-
Disulfide-reduced ALS variants of Cu, Zn superoxide dismutase exhibit increased populations of unfolded species.J Mol Biol. 2010 Apr 30;398(2):320-31. doi: 10.1016/j.jmb.2010.02.034. Epub 2010 Feb 23. J Mol Biol. 2010. PMID: 20184893 Free PMC article.
References
-
- Kikuchi H., Almer G., Yamashita S., Guegan C., Nagai M., Xu Z., Sosunov A.A., McKhann G.M., II, Przedborski S. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc. Natl Acad. Sci. USA. 2006;103:6025–6030. - PMC - PubMed
-
- Jaarsma D., Rognoni F., van Duijn W., Verspaget H.W., Haasdijk E.D., Holstege J.C. CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations. Acta Neuropathol. (Berl.) 2001;102:293–305. - PubMed
-
- Okado-Matsumoto A., Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J. Biol. Chem. 2001;276:38388–38393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

