Synergistic up-regulation of prostaglandin E synthase expression in breast cancer cells by 17beta-estradiol and proinflammatory cytokines
- PMID: 18703630
- PMCID: PMC6285349
- DOI: 10.1210/en.2008-0352
Synergistic up-regulation of prostaglandin E synthase expression in breast cancer cells by 17beta-estradiol and proinflammatory cytokines
Abstract
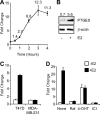
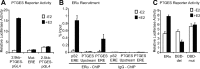
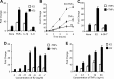
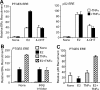
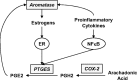
Inflammatory mediators, such as cytokines and prostaglandins, play a fundamental role in estrogen-dependent breast cancer through their ability to up-regulate aromatase expression and subsequent local production of estrogens in the breast. To study the link between estrogens and inflammation further, we examined the regulation of prostaglandin E synthase (PTGES), a key enzyme in the production of prostaglandin E2. We found that 17beta-estradiol (E2) rapidly and robustly up-regulates PTGES mRNA and protein levels in estrogen receptor (ER)-positive breast cancer cells through ER recruitment to an essential estrogen response element located in the 5' flanking region of the PTGES gene. PTGES is also up-regulated by the proinflammatory cytokines TNFalpha or IL-1beta. Surprisingly, the combination of E2 and cytokines leads to a synergistic up-regulation of PTGES in an ER and nuclear factor-kappaB (NFkappaB)-dependent manner. This is in contrast to the mutual transrepression between ER and NFkappaB that has been well characterized in other cell types. Furthermore, we found enhanced recruitment of ERalpha as well as the NFkappaB family member, p65, to the PTGES estrogen response element by the combination of E2 and TNFalpha compared with either E2 or TNFalpha alone. The synergistic up-regulation of PTGES may result in enhanced prostaglandin E2 production, which in turn may further enhance aromatase expression and production of local estrogens. Our findings suggest that a finely tuned positive feedback mechanism between estrogens and inflammatory factors may exist in the breast and contribute to hormone-dependent breast cancer growth and progression.
Figures


References
-
- Cicatiello L, Scafoglio C, Altucci L, Cancemi M, Natoli G, Facchiano A, Iazzetti G, Calogero R, Biglia N, De Bortoli M, Sfiligoi C, Sismondi P, Bresciani F, Weisz A. 2004. A genomic view of estrogen actions in human breast cancer cells by expression profiling of the hormone-responsive transcriptome. J Mol Endocrinol 32:719–775 - PubMed
-
- Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. 2003. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562–4574 - PubMed
-
- Inoue A, Yoshida N, Omoto Y, Oguchi S, Yamori T, Kiyama R, Hayashi S. 2002. Development of cDNA microarray for expression profiling of estrogen-responsive genes. J Mol Endocrinol 29:175–192 - PubMed
-
- Wang DY, Fulthorpe R, Liss SN, Edwards EA. 2004. Identification of estrogen-responsive genes by complementary deoxyribonucleic acid microarray and characterization of a novel early estrogen-induced gene: EEIG1. Mol Endocrinol 18:402–411 - PubMed
-
- Stender JD, Frasor J, Komm B, Chang KC, Kraus WL, Katzenellenbogen BS. 2007. Estrogen-regulated gene networks in human breast cancer cells: involvement of E2F1 in the regulation of cell proliferation. Mol Endocrinol 21:2112–2123 - PubMed

