Conformational switch of syntaxin-1 controls synaptic vesicle fusion
- PMID: 18703708
- PMCID: PMC3235364
- DOI: 10.1126/science.1163174
Conformational switch of syntaxin-1 controls synaptic vesicle fusion
Abstract
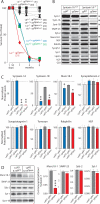
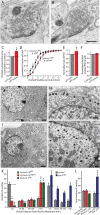
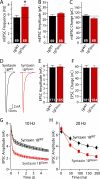
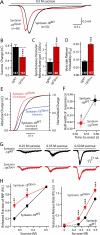
During synaptic vesicle fusion, the soluble N-ethylmaleimide-sensitive factor-attachment protein receptor (SNARE) protein syntaxin-1 exhibits two conformations that both bind to Munc18-1: a "closed" conformation outside the SNARE complex and an "open" conformation in the SNARE complex. Although SNARE complexes containing open syntaxin-1 and Munc18-1 are essential for exocytosis, the function of closed syntaxin-1 is unknown. We generated knockin/knockout mice that expressed only open syntaxin-1B. Syntaxin-1B(Open) mice were viable but succumbed to generalized seizures at 2 to 3 months of age. Binding of Munc18-1 to syntaxin-1 was impaired in syntaxin-1B(Open) synapses, and the size of the readily releasable vesicle pool was decreased; however, the rate of synaptic vesicle fusion was dramatically enhanced. Thus, the closed conformation of syntaxin-1 gates the initiation of the synaptic vesicle fusion reaction, which is then mediated by SNARE-complex/Munc18-1 assemblies.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

