In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies
- PMID: 18703742
- PMCID: PMC2606057
- DOI: 10.1126/science.1159194
In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies
Erratum in
- Science. 2008 Dec 12;322(5908):1634
Abstract
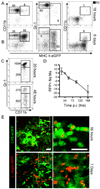
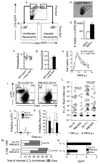
Infection with the obligate intracellular protozoan Leishmania is thought to be initiated by direct parasitization of macrophages, but the early events following transmission to the skin by vector sand flies have been difficult to examine directly. Using dynamic intravital microscopy and flow cytometry, we observed a rapid and sustained neutrophilic infiltrate at localized sand fly bite sites. Invading neutrophils efficiently captured Leishmania major (L.m.) parasites early after sand fly transmission or needle inoculation, but phagocytosed L.m. remained viable and infected neutrophils efficiently initiated infection. Furthermore, neutrophil depletion reduced, rather than enhanced, the ability of parasites to establish productive infections. Thus, L.m. appears to have evolved to both evade and exploit the innate host response to sand fly bite in order to establish and promote disease.
Figures


Comment in
-
Immunology. Neutrophil soldiers or Trojan Horses?Science. 2008 Aug 15;321(5891):917-8. doi: 10.1126/science.1162914. Science. 2008. PMID: 18703727 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

