Coenzyme Q1 redox metabolism during passage through the rat pulmonary circulation and the effect of hyperoxia
- PMID: 18703762
- PMCID: PMC2576032
- DOI: 10.1152/japplphysiol.00177.2008
Coenzyme Q1 redox metabolism during passage through the rat pulmonary circulation and the effect of hyperoxia
Abstract
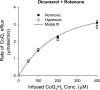
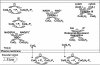
The objective was to evaluate the pulmonary disposition of the ubiquinone homolog coenzyme Q(1) (CoQ(1)) on passage through lungs of normoxic (exposed to room air) and hyperoxic (exposed to 85% O(2) for 48 h) rats. CoQ(1) or its hydroquinone (CoQ(1)H(2)) was infused into the arterial inflow of isolated, perfused lungs, and the venous efflux rates of CoQ(1)H(2) and CoQ(1) were measured. CoQ(1)H(2) appeared in the venous effluent when CoQ(1) was infused, and CoQ(1) appeared when CoQ(1)H(2) was infused. In normoxic lungs, CoQ(1)H(2) efflux rates when CoQ(1) was infused decreased by 58 and 33% in the presence of rotenone (mitochondrial complex I inhibitor) and dicumarol [NAD(P)H-quinone oxidoreductase 1 (NQO1) inhibitor], respectively. Inhibitor studies also revealed that lung CoQ(1)H(2) oxidation was via mitochondrial complex III. In hyperoxic lungs, CoQ(1)H(2) efflux rates when CoQ(1) was infused decreased by 23% compared with normoxic lungs. Based on inhibitor effects and a kinetic model, the effect of hyperoxia could be attributed predominantly to 47% decrease in the capacity of complex I-mediated CoQ(1) reduction, with no change in the other redox processes. Complex I activity in lung homogenates was also lower for hyperoxic than for normoxic lungs. These studies reveal that lung complexes I and III and NQO1 play a dominant role in determining the vascular concentration and redox status of CoQ(1) during passage through the pulmonary circulation, and that exposure to hyperoxia decreases the overall capacity of the lung to reduce CoQ(1) to CoQ(1)H(2) due to a depression in complex I activity.
Figures
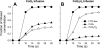



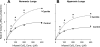

Similar articles
-
Coenzyme Q(1) as a probe for mitochondrial complex I activity in the intact perfused hyperoxia-exposed wild-type and Nqo1-null mouse lung.Am J Physiol Lung Cell Mol Physiol. 2012 May 1;302(9):L949-58. doi: 10.1152/ajplung.00251.2011. Epub 2012 Jan 20. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22268123 Free PMC article.
-
Role of mitochondrial electron transport complex I in coenzyme Q1 reduction by intact pulmonary arterial endothelial cells and the effect of hyperoxia.Am J Physiol Lung Cell Mol Physiol. 2007 Sep;293(3):L809-19. doi: 10.1152/ajplung.00448.2006. Epub 2007 Jun 29. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17601793
-
Differential responses of targeted lung redox enzymes to rat exposure to 60 or 85% oxygen.J Appl Physiol (1985). 2011 Jul;111(1):95-107. doi: 10.1152/japplphysiol.01451.2010. Epub 2011 May 5. J Appl Physiol (1985). 2011. PMID: 21551015 Free PMC article.
-
Idebenone and neuroprotection: antioxidant, pro-oxidant, or electron carrier?J Bioenerg Biomembr. 2015 Apr;47(1-2):111-8. doi: 10.1007/s10863-014-9571-y. Epub 2014 Sep 28. J Bioenerg Biomembr. 2015. PMID: 25262284 Free PMC article. Review.
-
The mitochondrial coenzyme Q junction and complex III: biochemistry and pathophysiology.FEBS J. 2022 Nov;289(22):6936-6958. doi: 10.1111/febs.16164. Epub 2021 Aug 30. FEBS J. 2022. PMID: 34428349 Review.
Cited by
-
Role of glutathione in lung retention of 99mTc-hexamethylpropyleneamine oxime in two unique rat models of hyperoxic lung injury.J Appl Physiol (1985). 2012 Aug 15;113(4):658-65. doi: 10.1152/japplphysiol.00441.2012. Epub 2012 May 24. J Appl Physiol (1985). 2012. PMID: 22628374 Free PMC article.
-
Optical imaging of tissue mitochondrial redox state in intact rat lungs in two models of pulmonary oxidative stress.J Biomed Opt. 2012 Apr;17(4):046010. doi: 10.1117/1.JBO.17.4.046010. J Biomed Opt. 2012. PMID: 22559688 Free PMC article.
-
Distribution of capillary transit times in isolated lungs of oxygen-tolerant rats.Ann Biomed Eng. 2010 Nov;38(11):3449-65. doi: 10.1007/s10439-010-0092-5. Epub 2010 Jun 15. Ann Biomed Eng. 2010. PMID: 20552277 Free PMC article.
-
Differential lung uptake of 99mTc-hexamethylpropyleneamine oxime and 99mTc-duramycin in the chronic hyperoxia rat model.J Nucl Med. 2012 Dec;53(12):1984-91. doi: 10.2967/jnumed.112.108498. Epub 2012 Oct 19. J Nucl Med. 2012. PMID: 23086010 Free PMC article.
-
Coenzyme Q(1) as a probe for mitochondrial complex I activity in the intact perfused hyperoxia-exposed wild-type and Nqo1-null mouse lung.Am J Physiol Lung Cell Mol Physiol. 2012 May 1;302(9):L949-58. doi: 10.1152/ajplung.00251.2011. Epub 2012 Jan 20. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22268123 Free PMC article.
References
-
- Anusevicius Z, Sarlauskas J, Cenas N. Two-electron reduction of quinones by rat liver NAD(P)H:quinone oxidoreductase: quantitative structure-activity relationships. Arch Biochem Biophys 404: 254–262, 2002. - PubMed
-
- Audi SH, Bongard RD, Dawson CA, Siegel D, Roerig DL, Merker MP. Duroquinone reduction during passage through the pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 285: L1116–L1131, 2003. - PubMed
-
- Audi SH, Bongard RD, Krenz GS, Rickaby DA, Haworth ST, Eisenhauer J, Roerig DL, Merker MP. Effect of chronic hyperoxic exposure on duroquinone reduction in adult rat lungs. Am J Physiol Lung Cell Mol Physiol 289: L788–L797, 2005. - PubMed
-
- Audi SH, Bongard RD, Okamoto Y, Merker MP, Roerig DL, Dawson CA. Pulmonary reduction of an intravascular redox polymer. Am J Physiol Lung Cell Mol Physiol 280: L1290–L1299, 2001. - PubMed
-
- Audi SH, Dawson CA, Ahlf SB, Roerig DL. Oxygen dependency of monoamine oxidase activity in the intact lung. Am J Physiol Lung Cell Mol Physiol 281: L969–L981, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

