Galactomannan antigenemia in pediatric oncology patients with invasive aspergillosis
- PMID: 18703991
- PMCID: PMC7006232
- DOI: 10.1097/INF.0b013e31817197ab
Galactomannan antigenemia in pediatric oncology patients with invasive aspergillosis
Abstract
Background: Diagnosing invasive aspergillosis is difficult but might be improved by detection of circulating galactomannan. Although galactomannan antigenemia has been well studied in the detection of invasive aspergillosis in adult patients, little is known about the expression of circulating galactomannan in immunocompromised children with invasive aspergillosis.
Methods: We studied the expression of galactomannan antigen by enzyme immunoassay (EIA) in 990 serum samples from 56 pediatric oncology patients (ages 3 months to 18 years) of whom 17 had proven or probable invasive aspergillosis defined by the European Organization for Research and Treatment of Cancer-Mycoses Study Group criteria. Any sample with a galactomannan EIA Galactomannan index value of > or = 0.5 was considered positive.
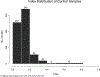
Results: At least 1 serum sample was positive for 11 of 17 pediatric oncology patients (65.7% sensitivity, 95% confidence interval: 38.3-85.7) with invasive aspergillosis. Galactomannan EIA was positive in 99 of 304 samples from patients with proven or probable invasive aspergillosis, and 7 of 686 (1.0%) samples from 39 control subjects resulted in a positive galactomannan EIA result. At least 1 sample tested positive in 5 of the 39 controls (12.8%, 95% confidence interval: 4.3-27.4). No significant association between accuracy and patient age was observed. Among the 7 evaluable galactomannan-positive patients with IA, the galactomannan EIA produced a positive result before clinical or radiographic evidence of infection in 6 cases, with a lead-time to diagnosis ranging from 1 day to 34 days (median: 10 days). In the remaining case, a positive galactomannan was observed on the same day as diagnosis by non-EIA methods.
Conclusions: The presence of circulating galactomannan is predictive of invasive aspergillosis in most pediatric oncology patients. Galactomannan antigenemia may precede clinical, microbiologic, or radiographic evidence of invasive aspergillosis.
Figures
References
-
- Benjamin DK Jr, Miller WC, Bayliff S, Martel L, Alexander KA, Martin PL. Infections diagnosed in the first year after pediatric stem cell transplantation. Pediatr Infect Dis J. 2002;21:227–234. - PubMed
-
- Groll AH, Kurz M, Schneider W, et al. Five-year-survey of invasive aspergillosis in a paediatric cancer centre. Epidemiology, management and long-term survival. Mycoses. 1999;42:431–442. - PubMed
-
- Steinbach WJ, Walsh TJ. Mycoses in pediatric patients. Infect Dis Clin North Am. 2006;20:663–678. - PubMed
-
- Almyroudis NG, Holland SM, Segal BH. Invasive aspergillosis in primary immunodeficiencies. Med Mycol. 2005;43(Suppl 1):S247–S259. - PubMed
-
- Robinson MR, Fine HF, Ross ML, et al. Sino-orbital-cerebral aspergillosis in immunocompromised pediatric patients: a report of three cases and review. Pediatr Infect Dis J. 2001;19:1197–1203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

