Hsp104 antagonizes alpha-synuclein aggregation and reduces dopaminergic degeneration in a rat model of Parkinson disease
- PMID: 18704197
- PMCID: PMC2515383
- DOI: 10.1172/JCI35781
Hsp104 antagonizes alpha-synuclein aggregation and reduces dopaminergic degeneration in a rat model of Parkinson disease
Abstract
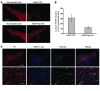
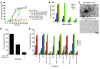
Parkinson disease (PD) is characterized by dopaminergic neurodegeneration and intracellular inclusions of alpha-synuclein amyloid fibers, which are stable and difficult to dissolve. Whether inclusions are neuroprotective or pathological remains controversial, because prefibrillar oligomers may be more toxic than amyloid inclusions. Thus, whether therapies should target inclusions, preamyloid oligomers, or both is a critically important issue. In yeast, the protein-remodeling factor Hsp104 cooperates with Hsp70 and Hsp40 to dissolve and reactivate aggregated proteins. Metazoans, however, have no Hsp104 ortholog. Here we introduced Hsp104 into a rat PD model. Remarkably, Hsp104 reduced formation of phosphorylated alpha-synuclein inclusions and prevented nigrostriatal dopaminergic neurodegeneration induced by PD-linked alpha-synuclein (A30P). An in vitro assay employing pure proteins revealed that Hsp104 prevented fibrillization of alpha-synuclein and PD-linked variants (A30P, A53T, E46K). Hsp104 coupled ATP hydrolysis to the disassembly of preamyloid oligomers and amyloid fibers composed of alpha-synuclein. Furthermore, the mammalian Hsp70 and Hsp40 chaperones, Hsc70 and Hdj2, enhanced alpha-synuclein fiber disassembly by Hsp104. Hsp104 likely protects dopaminergic neurons by antagonizing toxic alpha-synuclein assemblies and might have therapeutic potential for PD and other neurodegenerative amyloidoses.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

