Outcomes of the POG 9340/9341/9342 trials for children with high-risk neuroblastoma: a report from the Children's Oncology Group
- PMID: 18704922
- PMCID: PMC4426094
- DOI: 10.1002/pbc.21713
Outcomes of the POG 9340/9341/9342 trials for children with high-risk neuroblastoma: a report from the Children's Oncology Group
Abstract
Background: From 1993 to 1995, the Pediatric Oncology Group (POG) enrolled patients with high-risk neuroblastoma on three sequential, conjoined studies: a phase II induction window (9340), followed by intensive multiagent induction chemotherapy (9341), and subsequent myeloablative therapy with autologous stem cell rescue (9342). We report here the outcomes of patients treated on these studies.
Patients and methods: Patients were between 1 and 21 years old with high-risk neuroblastoma. Phase II window therapy consisted of two courses of either paclitaxel, topotecan, or cyclophosphamide with topotecan. Induction therapy consisted of at least five cycles of intensive chemotherapy, followed by myeloablative therapy with purged autologous stem cell reinfusion. Patient responses, treatment toxicities, and overall and event-free survival rates were calculated.
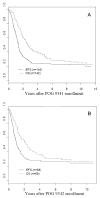
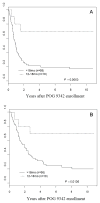
Results: Eighty-four percent of patients responded to induction chemotherapy, with 39% achieving complete response. Toxicities were primarily hematologic. The 7-year EFS and OS rates for all eligible patients on POG 9341 were 23 +/- 4% and 28 +/- 4%, respectively. The 7-year EFS and OS rates for patients treated on POG 9342 were 27 +/- 6% and 29 +/- 6%, respectively.
Conclusions: These studies were the first attempt by POG to use autologous stem cell transplantation for neuroblastoma treatment in a cooperative group setting. Toxicities and outcomes were comparable to contemporary cooperative group studies. The phase II induction window had no detectable effect on outcomes. New strategies are needed to improve survival for this devastating disease.
Figures

Comment in
-
High risk neuroblastoma: a persistent therapeutic challenge.Pediatr Blood Cancer. 2008 Dec;51(6):722-3. doi: 10.1002/pbc.21754. Pediatr Blood Cancer. 2008. PMID: 18819123 No abstract available.
References
-
- Cheung NV, Heller G. Chemotherapy Dose Intensity Correlates Strongly with Response, Median Survival, and Median Progression-Free Survival in Metastatic Neuroblastoma. J Clin Oncol. 1991;9:1050–8. - PubMed
-
- Ladenstein R, Philip T, Lasset C, et al. Multivariate Analysis of Risk Factors in Stage 4 Neuroblastoma Patients Over the Age of One Year Treated with Megatherapy and Stem Cell Transplantation: A Report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol. 1998;16:953–65. - PubMed
-
- Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of High-Risk Neuroblastoma with Intensive Chemotherapy, Radiotherapy, Autologous Bone Marrow Transplantation, and 13-cis-Retinoic acid. New Engl J Med. 1999;341:1165–73. - PubMed
-
- Frappaz D, Michon J, Coze C, et al. LMCE3 Treatment Strategy: Results in 99 Consecutively Diagnosed Stage 4 Neuroblastomas in Children Older than 1 Year at Diagnosis. J Clin Oncol. 2000;18:468–76. - PubMed
-
- Kaneko M, Tsuchida Y, Mugishima H, et al. Intensified Chemotherapy Increases the Survival Rates in Patients with Stage 4 Neuroblastoma with MYCN Amplification. J Pediatr Hematol Oncol. 2002;24:613–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

