Patient assessment of a novel therapeutic approach for the treatment of severe, chronic pain
- PMID: 18705820
- PMCID: PMC2658020
- DOI: 10.1111/j.1742-1241.2008.01820.x
Patient assessment of a novel therapeutic approach for the treatment of severe, chronic pain
Abstract
Background and objectives: Opioid-induced constipation can have a major negative impact on patients' quality of life. This randomised clinical trial evaluated patient assessment of the efficacy and tolerability of oral prolonged-release (PR) oxycodone when co-administered with oral naloxone PR.
Methods: Two hundred and two patients with chronic cancer- or non-cancer-related pain undergoing stable oxycodone PR therapy (40, 60 or 80 mg/day) were randomised to one of four intervention groups: 10, 20 or 40 mg/day naloxone PR or placebo. Following a 4-week maintenance phase, patients were followed-up for 2 weeks in which time they received oxycodone PR only. At the end of the maintenance phase, patients and investigators were asked to assess treatment efficacy and tolerability, as well as preference for the titration or maintenance phase.
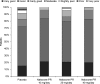
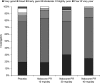
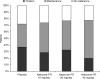
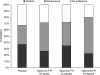
Results: Patient and investigator global assessment of efficacy and tolerability improved with increasing naloxone dose. Efficacy was ranked as 'good' or 'very good' by 50.0%, 67.4% and 72.5% of patients in the 10, 20 and 40 mg naloxone PR dose groups, respectively, compared with 43.5% of patients in the placebo group. Patient assessment of tolerability was similar between treatment groups and placebo, being ranked as 'good' or 'very good' by 83.3%, 79.1% and 82.5% of patients in the 10, 20 and 40 mg/day naloxone PR dose groups, respectively, compared with 71.7% of patients in the placebo group. The maintenance treatment phase was preferred by patients in the naloxone groups. A 2 : 1 dose ratio of oxycodone to naloxone was also assessed. Efficacy was ranked as 'good' or 'very good' by 70.4% of patients treated with the 2 : 1 dose ratio compared with 43.5% of patients receiving placebo. Tolerability of the 2 : 1 dose ratio was ranked as being 'good' or 'very good' by 81.5% of patients compared with 71.1% for the placebo group and patients preferred the maintenance phase.
Conclusions: The co-administration of oral naloxone PR with oxycodone PR improves patient assessment of analgesic opioid therapy for severe chronic pain, in terms of both efficacy and tolerability.
Figures



References
-
- Coluzzi F, Mattia C. Oxycodone. Pharmacological profile and clinical data in chronic pain management. Minerva Anestesiol. 2005;71:451–60. - PubMed
-
- Kalso E. Oxycodone. J Pain. 2005;29:S47–56. - PubMed
-
- Hale ME, Fleischmann R, Salzman R, et al. Efficacy and safety of controlled-release versus immediate-release oxycodone: randomized, double-blind evaluation in patients with chronic back pain. Clin J Pain. 1999;15:179–83. - PubMed
-
- Wirz S, Wartenberg HC, Wittman M. Post-operative pain therapy with controlled release oxycodone or controlled release tramadol following orthopedic surgery: a prospective, randomized, double-blind investigation. Pain Clin. 2005;17:367–76.
-
- Gimbel JS, Richards P, Portenoy RK. Controlled-release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology. 2003;60:927–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

