The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat
- PMID: 18705859
- PMCID: PMC6640518
- DOI: 10.1111/j.1364-3703.2008.00475.x
The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat
Abstract
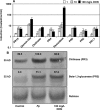
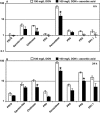
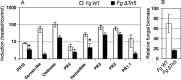
Fusarium species infect cereal crops worldwide and cause the important diseases Fusarium head blight and crown rot in wheat. Fusarium pathogens reduce yield and some species also produce trichothecene mycotoxins, such as deoxynivalenol (DON), during infection. These toxins play roles in pathogenesis on wheat and have serious health effects if present in grain consumed by humans or animals. In the present study, the response of wheat tissue to DON has been investigated. Infusion of wheat leaves with DON induced hydrogen peroxide production within 6 h followed by cell death within 24 h that was accompanied by DNA laddering, a hallmark of programmed cell death. In addition, real-time PCR analysis revealed that DON treatment rapidly induced transcription of a number of defence genes in a concentration-dependent manner. Co-treatment with DON and the antioxidant ascorbic acid reduced these responses, suggesting their induction may be at least partially mediated by reactive oxygen species (ROS), commonly known to be signalling molecules in plants. Wheat defence genes were more highly expressed in wheat stems inoculated with a DON-producing fungal strain than those inoculated with a DON-non-producing mutant, but only at a late stage of infection. Taken together, the results are consistent with a model in which DON production during infection of wheat induces ROS, which on the one hand may stimulate programmed host cell death assisting necrotrophic fungal growth, whereas, on the other hand, the ROS may contribute to the induction of antimicrobial host defences.
Figures


References
-
- Akinsanmi, O.A. , Backhouse, D. , Simpfendorfer, S. and Chakraborty, S. (2006) Genetic diversity of Australian Fusarium graminearum and F. pseudograminearum . Plant Pathol. 55, 494–504.
-
- Akinsanmi, O.A. , Mitter, V. , Simpfendorfer, S. , Backhouse, D. and Chakraborty, S. (2004) Identity and pathogenicity of Fusarium spp. isolated from wheat fields in Queensland and northern New South Wales. Aust. J. Agr. Res. 55, 97–107.
-
- Ansari, K.I. , Walter, S. , Brennan, J.M. , Lemmens, M. , Kessans, S. , McGahern, A. , Egan, D. and Doohan, F.M. (2007) Retrotransposon and gene activation in wheat in response to mycotoxigenic and non‐mycotoxigenic‐associated Fusarium stress. Theor. Appl. Genet. 114, 927–937. - PubMed
-
- Bai, G.H. , Desjardins, A.E. and Plattner, R.D. (2002) Deoxynivalenol‐nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia, 153, 91–98. - PubMed
-
- Belenghi, B. , Acconcia, F. , Trovato, M. , Perazzolli, M. , Bocedi, A. , Polticelli, F. , Ascenzi, P. and Delledonne, M. (2003) AtCYS1, a cystatin from Arabidopsis thaliana, suppresses hypersensitive cell death. Eur. J. Biochem. 270, 2593–2604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

