Abscisic acid deficiency leads to rapid activation of tomato defence responses upon infection with Erwinia chrysanthemi
- PMID: 18705880
- PMCID: PMC6640284
- DOI: 10.1111/j.1364-3703.2007.00437.x
Abscisic acid deficiency leads to rapid activation of tomato defence responses upon infection with Erwinia chrysanthemi
Abstract
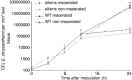
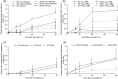
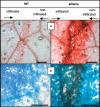
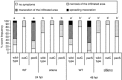
In addition to the important role of abscisic acid (ABA) in abiotic stress signalling, basal and high ABA levels appear to have a negative effect on disease resistance. Using the ABA-deficient sitiens tomato (Solanum lycopersicum) mutant and different application methods of exogenous ABA, we demonstrated the influence of this plant hormone on disease progression of Erwinia chrysanthemi. This necrotrophic plant pathogenic bacterium is responsible for soft rot disease on many plant species, causing maceration symptoms mainly due to the production and secretion of pectinolytic enzymes. On wild-type (WT) tomato cv. Moneymaker E. chrysanthemi leaf inoculation resulted in maceration both within and beyond the infiltrated zone of the leaf, but sitiens showed a very low occurrence of tissue maceration, which never extended the infiltrated zone. A single ABA treatment prior to infection eliminated the effect of pathogen restriction in sitiens, while repeated ABA spraying during plant development rendered both WT and sitiens very susceptible. Quantification of E. chrysanthemi populations inside the leaf did not reveal differences in bacterial growth between sitiens and WT. Sitiens was not more resistant to pectinolytic cell-wall degradation, but upon infection it showed a faster and stronger activation of defence responses than WT, such as hydrogen peroxide accumulation, peroxidase activation and cell-wall fortifications. Moreover, the rapid activation of sitiens peroxidases was also observed after application of bacteria-free culture filtrate containing E. chrysanthemi cell-wall-degrading enzymes and was absent during infection with an out E. chrysanthemi mutant impaired in secretion of these extracellular enzymes.
Figures



References
-
- Achuo, E.A. , Prinsen, E. and Höfte, M. (2006) Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici . Plant Pathol. 55, 178–186.
-
- Anderson, J.P. , Badruzsaufari, E. , Schenk, P.M. , Manners, J.M. , Desmond, O.J. , Ehlert, C. , Maclean, D.J. , Ebert, P.R. and Kazan, K. (2004) Antagonistic interaction between abscisic acid and jasmonate‐ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell, 16, 3460–3479. - PMC - PubMed
-
- Apel, K. and Hirt, H. (2004) Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 55, 373–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

