Methodology of the Interventional Management of Stroke III Trial
- PMID: 18706007
- PMCID: PMC3057361
- DOI: 10.1111/j.1747-4949.2008.00151.x
Methodology of the Interventional Management of Stroke III Trial
Abstract
Rationale: The Interventional Management of Stroke (IMS) I and II pilot trials demonstrated that the combined intravenous (i.v.) and intraarterial (i.a.) approach to recanalization may be more effective than standard i.v. rt-PA (Activase) alone for moderate-to-large National Institutes of Health Stroke Scale (NIHSS>or=10) strokes, and with a similar safety profile.
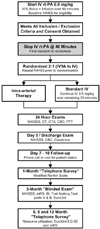
Aims: The primary objective of this NIH-funded, Phase III, randomized, multicenter, open-label clinical trial is to determine whether a combined i.v./i.a. approach to recanalization is superior to standard i.v. rt-PA alone when initiated within 3 h of acute ischemic stroke onset. The IMS III trial will develop and maintain a network of interventional centers to test the safety, feasibility, and potential efficacy of new FDA-approved catheter devices as part of a combined i.v./i.a. approach to recanalization as the IMS III study progresses. A secondary objective of the IMS III trial is to determine the cost-effectiveness of the combined i.v./i.a. approach as compared with standard i.v. rt-PA. Trial enrollment began in July of 2006.
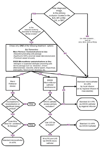
Design: A projected 900 subjects with moderate-to-large (NIHSS>or=10) ischemic strokes between ages 18 and 80 will be enrolled over the next 5 years at 40-plus centers in the United States and Canada. Patients must have i.v. treatment initiated within 3 h of stroke onset in both arms. Subjects will be randomized in a 2 : 1 ratio with more subjects enrolled in the combined i.v./i.a. group. The i.v. rt-PA alone group will receive the standard full dose [0.9 mg/kg, 90 mg maximum (10% as bolus)] of rt-PA intravenously over an hour. The combined i.v./i.a. group will receive a lower dose of i.v. rt-PA ( approximately 0.6 mg/kg, 60 mg maximum) over 40 min, followed by immediate angiography. If a treatable thrombus is not demonstrated, no i.a. therapy will be administered. If an appropriate thrombus is identified, treatment will continue with either the Concentric Merci thrombus-removal device, infusion of rt-PA and delivery of low-intensity ultrasound at the site of the occlusion via the EKOS Micro-Infusion Catheter, or infusion of rt-PA via a standard microcatheter. If i.a. rt-Pa therapy is the chosen strategy, a maximum of 22 mg of i.a. rt-PA may be given. The choice of i.a. strategy will be made by the treating neurointerventionalist. The i.a. treatment must begin within 5 h and be completed within 7 h of stroke onset.
Study outcomes: The primary outcome measure is a favorable clinical outcome, defined as a modified Rankin Scale Score of 0-2 at 3 months. The primary safety measure is mortality at 3 months and symptomatic ICH within the 24 h of randomization.
Conflict of interest statement
Funding sources are as above. There are no additional conflicts of interest.
Figures
References
-
- Brott TG, Haley EC, Jr, Levy DE, Barsan W, Broderick J, Sheppard GL, Spilker J, Kongable GL, Massey S, Reed R, et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke. 1992;23:632–640. - PubMed
-
- Haley E, Levy D, Brott T, Sheppard G, Wong M, Kongable G, Torner J, Marler J. Urgent therapy for stroke. Part II. Pilot study of tissue plasminogen activator administered 91–180 minutes from onset. Stroke. 1992;23:641–645. - PubMed
-
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. ATLANTIS Trials Investigators., ECASS Trials Investigators., NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. - PubMed
-
- NINDS rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical