Interferon-induced protein IFIT4 is associated with systemic lupus erythematosus and promotes differentiation of monocytes into dendritic cell-like cells
- PMID: 18706081
- PMCID: PMC2575605
- DOI: 10.1186/ar2475
Interferon-induced protein IFIT4 is associated with systemic lupus erythematosus and promotes differentiation of monocytes into dendritic cell-like cells
Abstract
Introduction: Using oligonucleotide microarray, many IFN-inducible genes have been found to be highly expressed in peripheral blood mononuclear cells (PBMCs) from most patients with systemic lupus erythematosus (SLE). Among these IFN-inducible genes, IFN-induced protein with tetratricopeptide repeats 4 (IFIT4) is a novel gene whose function is unknown.
Methods: In this study we examined the role played by IFIT4 in monocyte differentiation and the correlation between IFIT4 expression and the clinical manifestation of SLE. To this end, we used plasmid transfection, flow cytometry, mixed leucocyte responses, ELISA, quantitative RT-PCR and Western blotting.
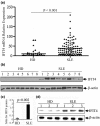
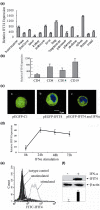
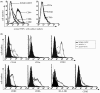
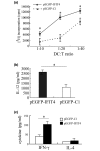
Results: We found that both IFIT4 mRNA and protein expression levels were significantly higher in PBMCs and monocytes from SLE patients than in those from healthy control individuals. IFIT4 expression was positively correlated with antinuclear antibodies, anti-double-stranded DNA, and anti-Sm auto-immune antibodies in SLE. Patients with SLE exhibiting higher expression of IFIT4 had a higher prevalence of leucopenia, thrombocytopenia and C3/C4 decrease. IFIT4 protein was localized exclusively to the cytoplasm, and it was significantly upregulated by IFN-alpha in normal PBMCs. To determine the role played by IFIT4 in monocyte differentiation, the monocytic cell line THP-1 was transfected with pEGFP-IFIT4 expression plasmid and stimulated with granulocyte-macrophage colony-stimulating factor/IL-4 to generate IFIT4-primed dendritic cell-like cells (DCLCs). IFIT4-primed DCLCs acquired morphological characteristics of dendritic cells more quickly, with greater resemblance to dendritic cells, as compared with DCLCs primed with pEGFP-C1 control plasmid trasfection. Furthermore, they exhibited higher expressions of CD40, CD86, CD80, HLA-DR and CD83, along with lower expression of CD14; increased IL-12 secretion; and an increased ability to stimulate T-cell proliferation. In addition, IFIT4-primed DCLCs enhanced IFN-gamma secretion (about 2.4-fold) by T cells compared with controls.
Conclusion: Our findings suggest that IFIT4 might play roles in promoting monocyte differentiation into DCLCs and in directing DCLCs to modulate T-helper-1 cell differentiation; these actions might contribute to the autoimmunity and pathogenesis of SLE.
Figures


Similar articles
-
Sequential activation of protein kinase C delta and JNK is required for interferon-alpha-induced expression of IFIT4.Cell Signal. 2008 Jan;20(1):112-9. doi: 10.1016/j.cellsig.2007.08.020. Epub 2007 Sep 26. Cell Signal. 2008. PMID: 17933493
-
Role of the cytokine environment and cytokine receptor expression on the generation of functionally distinct dendritic cells from human monocytes.Eur J Immunol. 2008 Mar;38(3):750-62. doi: 10.1002/eji.200737395. Eur J Immunol. 2008. PMID: 18236400
-
Expression of the markers BDCA-2 and BDCA-4 and production of interferon-alpha by plasmacytoid dendritic cells in systemic lupus erythematosus.Arthritis Rheum. 2003 Sep;48(9):2524-32. doi: 10.1002/art.11225. Arthritis Rheum. 2003. PMID: 13130472
-
Anti-interferon alpha treatment in SLE.Clin Immunol. 2013 Sep;148(3):303-12. doi: 10.1016/j.clim.2013.02.013. Epub 2013 Mar 1. Clin Immunol. 2013. PMID: 23566912 Review.
-
Interferon-α in the generation of monocyte-derived dendritic cells: recent advances and implications for dermatology.Br J Dermatol. 2011 Aug;165(2):247-54. doi: 10.1111/j.1365-2133.2011.10301.x. Epub 2011 Jun 2. Br J Dermatol. 2011. PMID: 21410666 Review.
Cited by
-
Identification of key interferon-stimulated genes for indicating the condition of patients with systemic lupus erythematosus.Front Immunol. 2022 Jul 28;13:962393. doi: 10.3389/fimmu.2022.962393. eCollection 2022. Front Immunol. 2022. PMID: 35967341 Free PMC article.
-
Clinical Values of the Identified Hub Genes in Systemic Lupus Erythematosus.Front Immunol. 2022 Jun 9;13:844025. doi: 10.3389/fimmu.2022.844025. eCollection 2022. Front Immunol. 2022. PMID: 35757684 Free PMC article.
-
Genome-Wide DNA Methylation Analysis of Chinese Patients with Systemic Lupus Erythematosus Identified Hypomethylation in Genes Related to the Type I Interferon Pathway.PLoS One. 2017 Jan 13;12(1):e0169553. doi: 10.1371/journal.pone.0169553. eCollection 2017. PLoS One. 2017. PMID: 28085900 Free PMC article.
-
Identification of unique microRNA signature associated with lupus nephritis.PLoS One. 2010 May 11;5(5):e10344. doi: 10.1371/journal.pone.0010344. PLoS One. 2010. PMID: 20485490 Free PMC article.
-
Common Marker Genes Identified from Various Sample Types for Systemic Lupus Erythematosus.PLoS One. 2016 Jun 3;11(6):e0156234. doi: 10.1371/journal.pone.0156234. eCollection 2016. PLoS One. 2016. PMID: 27257790 Free PMC article.
References
-
- Vakharia DD, Szebenyi SE, Gutterman JU, Rich SA. Interferon-alpha-induced human lupus inclusions and p36 protein in cancer and AIDS. J Interferon Cytokine Res. 1996;16:709–715. - PubMed
-
- Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. - DOI - PMC - PubMed
-
- Feng X, Wu H, Grossman JM, Hanvivadhanakul P, FitzGerald JD, Park GS, Dong X, Chen W, Kim MH, Weng HH, Furst DE, Gorn A, McMahon M, Taylor M, Brahn E, Hahn BH, Tsao BP. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2951–2962. doi: 10.1002/art.22044. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

