The chemical neuroanatomy of breathing
- PMID: 18706532
- PMCID: PMC2701569
- DOI: 10.1016/j.resp.2008.07.014
The chemical neuroanatomy of breathing
Abstract
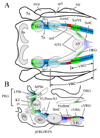
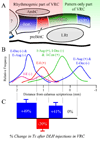
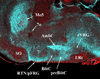
The chemical neuroanatomy of breathing must ultimately encompass all the various neuronal elements physiologically identified in brainstem respiratory circuits and their apparent aggregation into "compartments" within the medulla and pons. These functionally defined respiratory compartments in the brainstem provide the major source of input to cranial motoneurons controlling the airways, and to spinal motoneurons activating inspiratory and expiratory pump muscles. This review provides an overview of the neuroanatomy of the major compartments comprising brainstem respiratory circuits, and a synopsis of the transmitters used by their constituent respiratory neurons.
Figures

References
-
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. - PubMed
-
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol. 2004;143:105–114. - PubMed
-
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. - PubMed
-
- Averill DB, Cameron WE, Berger AJ. Neural elements subserving pulmonary stretch receptor-mediated facilitation of phrenic motoneurons. Brain Res. 1985;346:378–382. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

