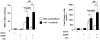Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI)
- PMID: 18706698
- PMCID: PMC3771643
- DOI: 10.1016/j.molimm.2008.07.010
Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI)
Abstract
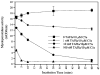
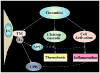
Thrombin-activatable procarboxypeptidase B (proCPB or thrombin-activatable fibrinolysis inhibitor or TAFI) is a plasma procarboxypeptidase that is activated by the thrombin-thrombomodulin complex on the vascular endothelial surface. The activated CPB removes the newly exposed carboxyl terminal lysines in the partially digested fibrin clot, diminishes tissue plasminogen activator and plasminogen binding, and protects the clot from premature lysis. We have recently shown that CPB is catalytically more efficient than plasma CPN, the major plasma anaphylatoxin inhibitor, in inhibiting bradykinin, activated complement C3a, C5a, and thrombin-cleaved osteopontin in vitro. Using a thrombin mutant (E229K) that has minimal procoagulant properties but retains the ability to activate protein C and proCPB in vivo, we showed that infusion of E229K thrombin into wild-type mice reduced bradykinin-induced hypotension but it had no effect in proCPB-deficient mice, indicating that the beneficial effect of E229K thrombin is mediated through its activation of proCPB and not protein C. Similarly proCPB-deficient mice displayed enhanced pulmonary inflammation in a C5a-induced alveolitis model and E229K thrombin ameliorated the magnitude of alveolitis in wild-type but not proCPB-deficient mice. ProCPB-deficient mice also displayed enhanced arthritis in an inflammatory arthritis model. Thus, our in vitro and in vivo data support the thesis that thrombin-activatable CPB has broad anti-inflammatory properties. By specific cleavage of the carboxyl terminal arginines from C3a, C5a, bradykinin and thrombin-cleaved osteopontin, it inactivates these active inflammatory mediators. Along with the activation of protein C, the activation of proCPB by the endothelial thrombin-thrombomodulin complex represents a homeostatic feedback mechanism in regulating thrombin's pro-inflammatory functions in vivo.
Figures
Similar articles
-
Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI).Adv Exp Med Biol. 2008;632:61-9. Adv Exp Med Biol. 2008. PMID: 19025114 Review.
-
Thrombin-activatable procarboxypeptidase B regulates activated complement C5a in vivo.Blood. 2007 Mar 1;109(5):1992-7. doi: 10.1182/blood-2006-03-012567. Epub 2006 Nov 14. Blood. 2007. PMID: 17105819 Free PMC article.
-
Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation.J Biol Chem. 2003 Dec 19;278(51):51059-67. doi: 10.1074/jbc.M306977200. Epub 2003 Oct 2. J Biol Chem. 2003. PMID: 14525995
-
Thrombin-activatable fibrinolysis inhibitor.Curr Med Chem. 2004 Sep;11(17):2335-48. doi: 10.2174/0929867043364586. Curr Med Chem. 2004. PMID: 15379716 Review.
-
Thrombin-activatable fibrinolysis inhibitor (TAFI, plasma procarboxypeptidase B, procarboxypeptidase R, procarboxypeptidase U).J Thromb Haemost. 2003 Jul;1(7):1566-74. doi: 10.1046/j.1538-7836.2003.00329.x. J Thromb Haemost. 2003. PMID: 12871292 Review.
Cited by
-
The role of thrombomodulin lectin-like domain in inflammation.J Biomed Sci. 2012 Mar 27;19(1):34. doi: 10.1186/1423-0127-19-34. J Biomed Sci. 2012. PMID: 22449172 Free PMC article. Review.
-
TAFI deficiency causes maladaptive vascular remodeling after hemophilic joint bleeding.JCI Insight. 2019 Oct 3;4(19):e128379. doi: 10.1172/jci.insight.128379. JCI Insight. 2019. PMID: 31465300 Free PMC article.
-
The group migration of Dictyostelium cells is regulated by extracellular chemoattractant degradation.Mol Biol Cell. 2009 Jul;20(14):3295-304. doi: 10.1091/mbc.e09-03-0223. Epub 2009 May 28. Mol Biol Cell. 2009. PMID: 19477920 Free PMC article.
-
C3a, C5a renal expression and their receptors are correlated to severity of IgA nephropathy.J Clin Immunol. 2014 Feb;34(2):224-32. doi: 10.1007/s10875-013-9970-6. Epub 2013 Dec 12. J Clin Immunol. 2014. PMID: 24327134
-
Carboxypeptidase U (TAFIa): a new drug target for fibrinolytic therapy?J Thromb Haemost. 2009 Dec;7(12):1962-71. doi: 10.1111/j.1538-7836.2009.03596.x. Epub 2009 Aug 28. J Thromb Haemost. 2009. PMID: 19719827 Free PMC article. Review.
References
-
- Bajzar L, Morser J, Nesheim M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J Biol Chem. 1996;271:16603–16608. - PubMed
-
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. - PubMed
-
- Broze G, Higuchi DA. Coagulation-dependent inhibition of fibrinolysis: role of carboxypeptidase-U and the premature lysis of clots from hemophilic plasma. Blood. 1996;88:3815–3823. - PubMed
-
- Campbell WD, Lazoura E, Okada N, Okada H. Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol Immunol. 2002;46:131–134. - PubMed
-
- Carter WJ, Myles T, Gibbs CS, Leung LL, Huntington JA. Crystal structure of anticoagulant thrombin variant E217K provides insights into thrombin allostery. J Biol Chem. 2004;279:26387–26394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials