The ghost in the machine: small GTPases as spatial regulators of exocytosis
- PMID: 18706813
- PMCID: PMC6521695
- DOI: 10.1016/j.tcb.2008.06.007
The ghost in the machine: small GTPases as spatial regulators of exocytosis
Abstract
Temporal and spatial regulation of membrane-trafficking events is crucial to both membrane identity and overall cell polarity. Small GTPases of the Rab, Ral and Rho protein families have been implicated as important regulators of vesicle docking and fusion events. This review focuses on how these GTPases interact with the exocyst complex, which is a multisubunit tethering complex involved in the regulation of cell-surface transport and cell polarity. The Rab and Ral GTPases are thought to function in exocyst assembly and vesicle-tethering processes, whereas the Rho family GTPases seem to function in the local activation of the exocyst complex to facilitate downstream vesicle-fusion events. The localized activation of the exocyst by Rho GTPases is likely to have an important role in spatial regulation of exocytosis.
Figures

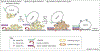

References
-
- Munson M and Novick P (2006) The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol 13, 577–581 - PubMed
-
- Hamburger ZA et al. (2006) Crystal structure of the S. cerevisiae exocyst component Exo70p. J. Mol. Biol 356, 9–21 - PubMed
-
- Whyte JR and Munro S (2001) The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell 1, 527–537 - PubMed
-
- Barrowman J and Novick P (2003) Three Yips for Rab recruitment. Nat. Cell Biol 5, 955–956 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

