Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors
- PMID: 18708183
- PMCID: PMC2668168
- DOI: 10.1016/j.biopsych.2008.07.005
Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors
Abstract
Background: Traumatic experiences may lead to debilitating psychiatric disorders including acute stress disorder and posttraumatic stress disorder. Current treatments for these conditions are largely ineffective, and novel therapies are needed. A cardinal symptom of these pathologies is the reexperiencing of the trauma through intrusive memories and nightmares. Studies in animal models indicate that memories can be weakened by interfering with the postretrieval restabilization process known as memory reconsolidation. We previously reported that, in rats, intraamygdala injection of the glucocorticoid receptor antagonist RU38486 disrupts the reconsolidation of a traumatic memory. Here we tested parameters important for designing novel clinical protocols targeting the reconsolidation of a traumatic memory with RU38486.
Methods: Using rat inhibitory avoidance, we tested the efficacy of postretrieval systemic administration of RU38486 on subsequent memory retention and evaluated several key preclinical parameters.
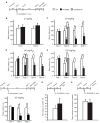
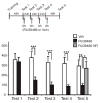
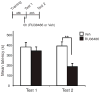
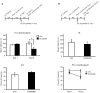
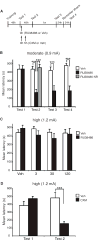
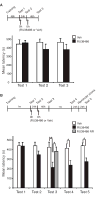
Results: Systemic administration of RU38486 before or after retrieval persistently weakens inhibitory avoidance memory retention in a dose-dependent manner, and memory does not reemerge following a footshock reminder. The efficacy of treatment is a function of the intensity of the initial trauma, and intense traumatic memories can be disrupted by changing the time and number of interventions. Furthermore, one or two treatments are sufficient to disrupt the memory maximally. The treatment selectively targets the reactivated memory without interfering with the retention of another nonreactivated memory.
Conclusions: RU38486 is a potential novel treatment for psychiatric disorders linked to traumatic memories. Our data provide the parameters for designing promising clinical trials for the treatment of flashback-type symptoms of PTSD.
Conflict of interest statement
The authors reported no biomedical financial interests or potential conflicts of interest.
Figures
Similar articles
-
Systemic and intrahippocampal administrations of the glucocorticoid receptor antagonist RU38486 impairs fear memory reconsolidation in rats.Stress. 2011 Jul;14(4):459-64. doi: 10.3109/10253890.2010.548171. Epub 2011 Mar 27. Stress. 2011. PMID: 21438774
-
Preclinical evaluation of reconsolidation blockade by clonidine as a potential novel treatment for posttraumatic stress disorder.Neuropsychopharmacology. 2012 Dec;37(13):2789-96. doi: 10.1038/npp.2012.145. Epub 2012 Aug 8. Neuropsychopharmacology. 2012. PMID: 22871915 Free PMC article.
-
From Memory Impairment to Posttraumatic Stress Disorder-Like Phenotypes: The Critical Role of an Unpredictable Second Traumatic Experience.J Neurosci. 2015 Dec 2;35(48):15903-15. doi: 10.1523/JNEUROSCI.0771-15.2015. J Neurosci. 2015. PMID: 26631471 Free PMC article.
-
Glucocorticoid-induced reduction of traumatic memories: implications for the treatment of PTSD.Prog Brain Res. 2008;167:239-47. doi: 10.1016/S0079-6123(07)67017-4. Prog Brain Res. 2008. PMID: 18037019 Review.
-
The role of glucocorticoids, catecholamines and endocannabinoids in the development of traumatic memories and posttraumatic stress symptoms in survivors of critical illness.Neurobiol Learn Mem. 2014 Jul;112:68-74. doi: 10.1016/j.nlm.2013.10.003. Epub 2013 Oct 11. Neurobiol Learn Mem. 2014. PMID: 24125890 Review.
Cited by
-
Limited replicability of drug-induced amnesia after contextual fear memory retrieval in rats.Neurobiol Learn Mem. 2019 Dec;166:107105. doi: 10.1016/j.nlm.2019.107105. Epub 2019 Nov 6. Neurobiol Learn Mem. 2019. PMID: 31705982 Free PMC article.
-
Windows of change: Revisiting temporal and molecular dynamics of memory reconsolidation and persistence.Neurosci Biobehav Rev. 2025 Jul;174:106198. doi: 10.1016/j.neubiorev.2025.106198. Epub 2025 May 10. Neurosci Biobehav Rev. 2025. PMID: 40354954 Review.
-
CGRP antagonist infused into the bed nucleus of the stria terminalis impairs the acquisition and expression of context but not discretely cued fear.Learn Mem. 2013 Nov 19;20(12):730-9. doi: 10.1101/lm.032482.113. Learn Mem. 2013. PMID: 24255102 Free PMC article.
-
The neurobiological bases of memory formation: from physiological conditions to psychopathology.Psychopathology. 2014;47(6):347-56. doi: 10.1159/000363702. Epub 2014 Oct 3. Psychopathology. 2014. PMID: 25301080 Free PMC article. Review.
-
CCAAT enhancer binding protein δ plays an essential role in memory consolidation and reconsolidation.J Neurosci. 2013 Feb 20;33(8):3646-58. doi: 10.1523/JNEUROSCI.1635-12.2013. J Neurosci. 2013. PMID: 23426691 Free PMC article.
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association Press; 2000.
-
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. - PubMed
-
- Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. Jama. 2000;283:1837–1844. - PubMed
-
- Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62:860–868. - PubMed
-
- Fairbank JA, Nicholson RA. Theoretical and empirical issues in the treatment of post-traumatic stress disorder in Vietnam veterans. J Clin Psychol. 1987;43:44–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

