Development of free-energy-based models for chaperonin containing TCP-1 mediated folding of actin
- PMID: 18708324
- PMCID: PMC2570749
- DOI: 10.1098/rsif.2008.0185
Development of free-energy-based models for chaperonin containing TCP-1 mediated folding of actin
Abstract
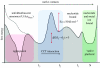
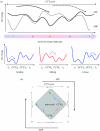
A free-energy-based approach is used to describe the mechanism through which chaperonin-containing TCP-1 (CCT) folds the filament-forming cytoskeletal protein actin, which is one of its primary substrates. The experimental observations on the actin folding and unfolding pathways are collated and then re-examined from this perspective, allowing us to determine the position of the CCT intervention on the actin free-energy folding landscape. The essential role for CCT in actin folding is to provide a free-energy contribution from its ATP cycle, which drives actin to fold from a stable, trapped intermediate I3, to a less stable but now productive folding intermediate I2. We develop two hypothetical mechanisms for actin folding founded upon concepts established for the bacterial type I chaperonin GroEL and extend them to the much more complex CCT system of eukaryotes. A new model is presented in which CCT facilitates free-energy transfer through direct coupling of the nucleotide hydrolysis cycle to the phases of actin substrate maturation.
Figures




References
-
- Altschuler, G. M. 2006 Protein folding studies on the actin–CCT chaperone system. PhD thesis, Institute of Cancer Research, University of London.
-
- Archibald J.M, O'Kelly C.J, Doolittle W.F. The chaperonin genes of jakobid and jakobid-like flagellates: implications for eukaryotic evolution. Mol. Biol. Evol. 2002;19:422–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

