Internalization of eNOS via caveolae regulates PAF-induced inflammatory hyperpermeability to macromolecules
- PMID: 18708444
- PMCID: PMC2593519
- DOI: 10.1152/ajpheart.00629.2008
Internalization of eNOS via caveolae regulates PAF-induced inflammatory hyperpermeability to macromolecules
Abstract
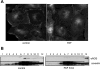
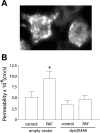
Endothelial nitric oxide (NO) synthase (eNOS) is thought to regulate microvascular permeability via NO production. We tested the hypotheses that the expression of eNOS and eNOS endocytosis by caveolae are fundamental for appropriate signaling mechanisms in inflammatory endothelial permeability to macromolecules. We used bovine coronary postcapillary venular endothelial cells (CVECs) because these cells are derived from the microvascular segment responsible for the transport of macromolecules in inflammation. We stimulated CVECs with platelet-activating factor (PAF) at 100 nM and measured eNOS phosphorylation, NO production, and CVEC monolayer permeability to FITC-dextran 70 KDa (Dx-70). PAF translocated eNOS from plasma membrane to cytosol, induced changes in the phosphorylation state of the enzyme, and increased NO production from 4.3+/-3.8 to 467+/-22.6 nM. PAF elevated CVEC monolayer permeability to FITC-Dx-70 from 3.4+/-0.3 x 10(-6) to 8.5+/-0.4 x 10(-6) cm/s. The depletion of endogenous eNOS with small interfering RNA abolished PAF-induced hyperpermeability, demonstrating that the expression of eNOS is required for inflammatory hyperpermeability responses. The inhibition of the caveolar internalization by blocking caveolar scission using transfection of dynamin dominant-negative mutant, dyn2K44A, inhibited PAF-induced hyperpermeability to FITC-Dx-70. We interpret these data as evidence that 1) eNOS is required for hyperpermeability to macromolecules and 2) the internalization of eNOS via caveolae is an important mechanism in the regulation of endothelial permeability. We advance the novel concept that eNOS internalization to cytosol is a signaling mechanism for the onset of microvascular hyperpermeability in inflammation.
Figures



References
-
- Bohlen HG Mechanism of increased vessel wall nitric oxide concentrations during intestinal absorption. Am J Physiol Heart Circ Physiol 275: H542–H550, 1998. - PubMed
-
- Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW 2nd, Durán WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol 284: H92–H100, 2003. - PubMed
-
- Cao S, Yao J, McCabe TJ, Yao Q, Katusic ZS, Sessa WC, Shah V. Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J Biol Chem 276: 14249–14256, 2001. - PubMed
-
- Chatterjee S, Cao S, Peterson TE, Simari RD, Shah V. Inhibition of GTP-dependent vesicle trafficking impairs internalization of plasmalemmal eNOS and cellular nitric oxide production. J Cell Sci 116: 3645–3655, 2003. - PubMed
-
- Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem 281: 1477–1488, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

