Molecular mechanism of ion-ion and ion-substrate coupling in the Na+-dependent leucine transporter LeuT
- PMID: 18708457
- PMCID: PMC2576368
- DOI: 10.1529/biophysj.108.139741
Molecular mechanism of ion-ion and ion-substrate coupling in the Na+-dependent leucine transporter LeuT
Abstract
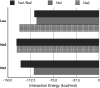
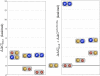
Ion-coupled transport of neurotransmitter molecules by neurotransmitter:sodium symporters (NSS) play an important role in the regulation of neuronal signaling. One of the major events in the transport cycle is ion-substrate coupling and formation of the high-affinity occluded state with bound ions and substrate. Molecular mechanisms of ion-substrate coupling and the corresponding ion-substrate stoichiometry in NSS transporters has yet to be understood. The recent determination of a high-resolution structure for a bacterial homolog of Na(+)/Cl(-)-dependent neurotransmitter transporters, LeuT, offers a unique opportunity to analyze the functional roles of the multi-ion binding sites within the binding pocket. The binding pocket of LeuT contains two metal binding sites. The first ion in site NA1 is directly coupled to the bound substrate (Leu) with the second ion in the neighboring site (NA2) only approximately 7 A away. Extensive, fully atomistic, molecular dynamics, and free energy simulations of LeuT in an explicit lipid bilayer are performed to evaluate substrate-binding affinity as a function of the ion load (single versus double occupancy) and occupancy by specific monovalent cations. It was shown that double ion occupancy of the binding pocket is required to ensure substrate coupling to Na(+) and not to Li(+) or K(+) cations. Furthermore, it was found that presence of the ion in site NA2 is required for structural stability of the binding pocket as well as amplified selectivity for Na(+) in the case of double ion occupancy.
Figures

Similar articles
-
Control of ion selectivity in LeuT: two Na+ binding sites with two different mechanisms.J Mol Biol. 2008 Mar 28;377(3):804-18. doi: 10.1016/j.jmb.2008.01.015. Epub 2008 Jan 15. J Mol Biol. 2008. PMID: 18280500 Free PMC article.
-
Conformational Dynamics on the Extracellular Side of LeuT Controlled by Na+ and K+ Ions and the Protonation State of Glu290.J Biol Chem. 2016 Sep 16;291(38):19786-99. doi: 10.1074/jbc.M116.731455. Epub 2016 Jul 29. J Biol Chem. 2016. PMID: 27474737 Free PMC article.
-
Molecular dynamics simulations of Na(+) and leucine transport by LeuT.Biochem Biophys Res Commun. 2015 Aug 14;464(1):281-5. doi: 10.1016/j.bbrc.2015.06.143. Epub 2015 Jun 24. Biochem Biophys Res Commun. 2015. PMID: 26116773
-
How LeuT shapes our understanding of the mechanisms of sodium-coupled neurotransmitter transporters.J Physiol. 2014 Mar 1;592(5):863-9. doi: 10.1113/jphysiol.2013.259051. Epub 2013 Jul 22. J Physiol. 2014. PMID: 23878376 Free PMC article. Review.
-
Cytoplasmic permeation pathway of neurotransmitter transporters.Biochemistry. 2011 Sep 6;50(35):7462-75. doi: 10.1021/bi200926b. Epub 2011 Aug 10. Biochemistry. 2011. PMID: 21774491 Free PMC article. Review.
Cited by
-
Arginine oscillation explains Na+ independence in the substrate/product antiporter CaiT.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17296-301. doi: 10.1073/pnas.1309071110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101465 Free PMC article.
-
Ion channels and ion selectivity.Essays Biochem. 2017 May 9;61(2):201-209. doi: 10.1042/EBC20160074. Print 2017 May 9. Essays Biochem. 2017. PMID: 28487397 Free PMC article. Review.
-
The RCK2 domain uses a coordination site present in Kir channels to confer sodium sensitivity to Slo2.2 channels.J Neurosci. 2010 Jun 2;30(22):7554-62. doi: 10.1523/JNEUROSCI.0525-10.2010. J Neurosci. 2010. PMID: 20519529 Free PMC article.
-
Chloride binding site of neurotransmitter sodium symporters.Proc Natl Acad Sci U S A. 2013 May 21;110(21):8489-94. doi: 10.1073/pnas.1221279110. Epub 2013 May 2. Proc Natl Acad Sci U S A. 2013. PMID: 23641004 Free PMC article.
-
Conformational Changes in Two Inter-Helical Loops of Mhp1 Membrane Transporter.PLoS One. 2015 Jul 17;10(7):e0133388. doi: 10.1371/journal.pone.0133388. eCollection 2015. PLoS One. 2015. PMID: 26186341 Free PMC article.
References
-
- Kanner, B. I., and E. Zomot. 2008. Sodium-coupled neurotransmitter transporters. Chem. Rev. 108:1654–1668. - PubMed
-
- DeFelice, L. J. 2004. Transporter structure and mechanism. Trends Neurosci. 27:352–359. - PubMed
-
- Gether, U., P. H. Andersen, O. M. Larsson, and A. Schousboe. 2006. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol. Sci. 27:375–383. - PubMed
-
- Rudnick, G. 2006. Serotonin transporters—structure and function. J. Membr. Biol. 213:101–110. - PubMed
-
- Kilic, F., D. L. Murphy, and G. Rudnick. 2003. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol. Pharmacol. 64:440–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

