Hydrogen bond switching among flavin and amino acid side chains in the BLUF photoreceptor observed by ultrafast infrared spectroscopy
- PMID: 18708458
- PMCID: PMC2576356
- DOI: 10.1529/biophysj.108.139246
Hydrogen bond switching among flavin and amino acid side chains in the BLUF photoreceptor observed by ultrafast infrared spectroscopy
Abstract
BLUF domains constitute a recently discovered class of photoreceptor proteins found in bacteria and eukaryotic algae. BLUF domains are blue-light sensitive through a FAD cofactor that is involved in an extensive hydrogen-bond network with nearby amino acid side chains, including a highly conserved tyrosine and glutamine. The participation of particular amino acid side chains in the ultrafast hydrogen-bond switching reaction with FAD that underlies photoactivation of BLUF domains is assessed by means of ultrafast infrared spectroscopy. Blue-light absorption by FAD results in formation of FAD(*-) and a bleach of the tyrosine ring vibrational mode on a picosecond timescale, showing that electron transfer from tyrosine to FAD constitutes the primary photochemistry. This interpretation is supported by the absence of a kinetic isotope effect on the fluorescence decay on H/D exchange. Subsequent protonation of FAD(*-) to result in FADH(*) on a picosecond timescale is evidenced by the appearance of a N-H bending mode at the FAD N5 protonation site and of a FADH(*) C=N stretch marker mode, with tyrosine as the likely proton donor. FADH(*) is reoxidized in 67 ps (180 ps in D(2)O) to result in a long-lived hydrogen-bond switched network around FAD. This hydrogen-bond switch shows infrared signatures from the C-OH stretch of tyrosine and the FAD C4=O and C=N stretches, which indicate increased hydrogen-bond strength at all these sites. The results support a previously hypothesized rotation of glutamine by approximately 180 degrees through a light-driven radical-pair mechanism as the determinant of the hydrogen-bond switch.
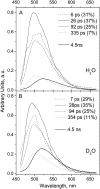
Figures





References
-
- Christie, J. M., and W. R. Briggs. 2005. Blue-light sensing and signaling by the phototropins. In Handbook of Photosensory Receptors. W. R. Briggs and J. L. Spudich, editors. Wiley-VCH Verlag GmbH & Company, Weinheim, Germany. 277–304.
-
- Crosson, S., S. Rajagopal, and K. Moffat. 2003. The LOV domain family: photoresponsive signaling modules coupled to diverse output domains. Biochemistry. 42:2–10. - PubMed
-
- Losi, A. 2006. Flavins: Photochemistry and Photobiology. E. Silvia and A. M. Edwards, editors. Royal Society of Chemistry, Cambridge, UK. 217–269.
-
- Swartz, T. E., S. B. Corchnoy, J. M. Christie, J. W. Lewis, I. Szundi, W. R. Briggs, and R. A. Bogomolni. 2001. The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J. Biol. Chem. 276:36493–36500. - PubMed
-
- Gomelsky, M., and G. Klug. 2002. BLUF: a novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem. Sci. 27:497–500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

