Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation
- PMID: 18708497
- PMCID: PMC2566190
- DOI: 10.1128/JB.00466-08
Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation
Abstract
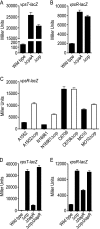
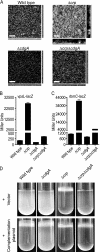
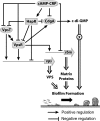
Vibrio cholerae is a facultative human pathogen. The ability of V. cholerae to form biofilms is crucial for its survival in aquatic habitats between epidemics and is advantageous for host-to-host transmission during epidemics. Formation of mature biofilms requires the production of extracellular matrix components, including Vibrio polysaccharide (VPS) and matrix proteins. Biofilm formation is positively controlled by the transcriptional regulators VpsR and VpsT and is negatively regulated by the quorum-sensing transcriptional regulator HapR, as well as the cyclic AMP (cAMP)-cAMP receptor protein (CRP) regulatory complex. Transcriptome analysis of cyaA (encoding adenylate cyclase) and crp (encoding cAMP receptor protein) deletion mutants revealed that cAMP-CRP negatively regulates transcription of both VPS biosynthesis genes and genes encoding biofilm matrix proteins. Further mutational and expression analysis revealed that cAMP-CRP negatively regulates transcription of vps genes indirectly through its action on vpsR transcription. However, negative regulation of the genes encoding biofilm matrix proteins by cAMP-CRP can also occur independent of VpsR. Transcriptome analysis also revealed that cAMP-CRP regulates the expression of a set of genes encoding diguanylate cyclases (DGCs) and phosphodiesterases. Mutational and phenotypic analysis of the differentially regulated DGCs revealed that a DGC, CdgA, is responsible for the increase in biofilm formation in the Deltacrp mutant, showing the connection between of cyclic di-GMP and cAMP signaling in V. cholerae.
Figures



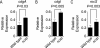
References
-
- Bao, Y., D. P. Lies, H. Fu, and G. P. Roberts. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109167-168. - PubMed
-
- Beyhan, S., and F. H. Yildiz. 2007. Smooth to rugose phase variation in Vibrio cholerae can be mediated by a single nucleotide change that targets c-di-GMP signalling pathway. Mol. Microbiol. 63995-1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

