Sinorhizobium meliloti mutants deficient in phosphatidylserine decarboxylase accumulate phosphatidylserine and are strongly affected during symbiosis with alfalfa
- PMID: 18708506
- PMCID: PMC2566212
- DOI: 10.1128/JB.00610-08
Sinorhizobium meliloti mutants deficient in phosphatidylserine decarboxylase accumulate phosphatidylserine and are strongly affected during symbiosis with alfalfa
Abstract
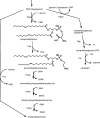
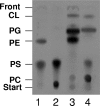
Sinorhizobium meliloti contains phosphatidylglycerol, cardiolipin, phosphatidylcholine, and phosphatidylethanolamine (PE) as major membrane lipids. PE is formed in two steps. In the first step, phosphatidylserine synthase (Pss) condenses serine with CDP-diglyceride to form phosphatidylserine (PS), and in the second step, PS is decarboxylated by phosphatidylserine decarboxylase (Psd) to form PE. In this study we identified the sinorhizobial psd gene coding for Psd. A sinorhizobial mutant deficient in psd is unable to form PE but accumulates the anionic phospholipid PS. Properties of PE-deficient mutants lacking either Pss or Psd were compared with those of the S. meliloti wild type. Whereas both PE-deficient mutants grew in a wild-type-like manner on many complex media, they were unable to grow on minimal medium containing high phosphate concentrations. Surprisingly, the psd-deficient mutant could grow on minimal medium containing low concentrations of inorganic phosphate, while the pss-deficient mutant could not. Addition of choline to the minimal medium rescued growth of the pss-deficient mutant, CS111, to some extent but inhibited growth of the psd-deficient mutant, MAV01. When the two distinct PE-deficient mutants were analyzed for their ability to form a nitrogen-fixing root nodule symbiosis with their alfalfa host plant, they behaved strikingly differently. The Pss-deficient mutant, CS111, initiated nodule formation at about the same time point as the wild type but did form about 30% fewer nodules than the wild type. In contrast, the PS-accumulating mutant, MAV01, initiated nodule formation much later than the wild type and formed 90% fewer nodules than the wild type. The few nodules formed by MAV01 seemed to be almost devoid of bacteria and were unable to fix nitrogen. Leaves of alfalfa plants inoculated with the mutant MAV01 were yellowish, indicating that the plants were starved for nitrogen. Therefore, changes in lipid composition, including the accumulation of bacterial PS, prevent the establishment of a nitrogen-fixing root nodule symbiosis.
Figures



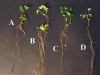
Similar articles
-
Phosphatidylethanolamine is not essential for growth of Sinorhizobium meliloti on complex culture media.J Bacteriol. 2004 Mar;186(6):1667-77. doi: 10.1128/JB.186.6.1667-1677.2004. J Bacteriol. 2004. PMID: 14996797 Free PMC article.
-
Phosphorus-free membrane lipids of Sinorhizobium meliloti are not required for the symbiosis with alfalfa but contribute to increased cell yields under phosphorus-limiting conditions of growth.Mol Plant Microbe Interact. 2005 Sep;18(9):973-82. doi: 10.1094/MPMI-18-0973. Mol Plant Microbe Interact. 2005. PMID: 16167767
-
Phosphatidylcholine-deficient suppressor mutant of Sinorhizobium meliloti, altered in fatty acid synthesis, partially recovers nodulation ability in symbiosis with alfalfa (Medicago sativa).Plant J. 2024 May;118(4):1136-1154. doi: 10.1111/tpj.16661. Epub 2024 Feb 11. Plant J. 2024. PMID: 38341846
-
How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model.Nat Rev Microbiol. 2007 Aug;5(8):619-33. doi: 10.1038/nrmicro1705. Nat Rev Microbiol. 2007. PMID: 17632573 Free PMC article. Review.
-
Membrane lipids in plant-associated bacteria: their biosyntheses and possible functions.Mol Plant Microbe Interact. 2003 Jul;16(7):567-79. doi: 10.1094/MPMI.2003.16.7.567. Mol Plant Microbe Interact. 2003. PMID: 12848422 Review.
Cited by
-
Mesorhizobium huakuii HtpG Interaction with nsLTP AsE246 Is Required for Symbiotic Nitrogen Fixation.Plant Physiol. 2019 May;180(1):509-528. doi: 10.1104/pp.18.00336. Epub 2019 Feb 14. Plant Physiol. 2019. PMID: 30765481 Free PMC article.
-
ExoS/ChvI Two-Component Signal-Transduction System Activated in the Absence of Bacterial Phosphatidylcholine.Front Plant Sci. 2021 Jul 23;12:678976. doi: 10.3389/fpls.2021.678976. eCollection 2021. Front Plant Sci. 2021. PMID: 34367203 Free PMC article.
-
Fatty acid-releasing activities in Sinorhizobium meliloti include unusual diacylglycerol lipase.Environ Microbiol. 2015 Sep;17(9):3391-406. doi: 10.1111/1462-2920.12814. Epub 2015 Mar 27. Environ Microbiol. 2015. PMID: 25711932 Free PMC article.
-
Members of the Sinorhizobium meliloti ChvI regulon identified by a DNA binding screen.BMC Microbiol. 2013 Jun 13;13:132. doi: 10.1186/1471-2180-13-132. BMC Microbiol. 2013. PMID: 23758731 Free PMC article.
-
Open Issues for Protein Function Assignment in Haloferax volcanii and Other Halophilic Archaea.Genes (Basel). 2021 Jun 24;12(7):963. doi: 10.3390/genes12070963. Genes (Basel). 2021. PMID: 34202810 Free PMC article.
References
-
- Baunaure, F., P. Eldin, A.-M. Cathiard, and H. Vial. 2004. Characterization of a non-mitochondrial type I phosphatidylserine decarboxylase in Plasmodium falciparum. Mol. Microbiol. 5133-46. - PubMed
-
- Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84188-198. - PubMed
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37911-917. - PubMed
-
- Bogdanov, M., and W. Dowhan. 1995. Phosphatidylethanolamine is required for in vivo function of the membrane-associated lactose permease in Escherichia coli. J. Biol. Chem. 270732-739. - PubMed
-
- Bogdanov, M., and W. Dowhan. 2002. Functional roles of lipids in membranes, p. 1-35. In D. E. Vance and J. E. Vance (ed.), Biochemistry of lipids, lipoproteins and membranes, 4th ed. Elsevier, Amsterdam, The Netherlands.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

