High and low temperatures differently affect infection density and vertical transmission of male-killing Spiroplasma symbionts in Drosophila hosts
- PMID: 18708518
- PMCID: PMC2565954
- DOI: 10.1128/AEM.01503-08
High and low temperatures differently affect infection density and vertical transmission of male-killing Spiroplasma symbionts in Drosophila hosts
Abstract
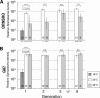
We investigated the vertical transmission, reproductive phenotype, and infection density of a male-killing Spiroplasma symbiont in two Drosophila species under physiological high and low temperatures through successive host generations. In both the native host Drosophila nebulosa and the nonnative host Drosophila melanogaster, the symbiont infection and the male-killing phenotype were stably maintained at 25 degrees C, rapidly lost at 18 degrees C, and gradually lost at 28 degrees C. In the nonnative host, both the high and low temperatures significantly suppressed the infection density of the spiroplasma. In the native host, by contrast, the low temperature suppressed the infection density of the spiroplasma whereas the high temperature had little effect on the infection density. These results suggested that the low temperature suppresses both the infection density and the vertical transmission of the spiroplasma whereas the high temperature suppresses the vertical transmission preferentially. The spiroplasma density was consistently higher in the native host than in the nonnative host, suggesting that the host genotype may affect the infection density of the symbiont. The temperature- and genotype-dependent instability of the symbiont infection highlights a complex genotype-by-genotype-by-environment interaction and may be relevant to the low infection frequencies of the male-killing spiroplasmas in natural Drosophila populations.
Figures



References
-
- Bourtzis, K., and T. A. Miller. 2003. Insect symbiosis. CRC Press, New York, NY.
-
- Bourtzis, K., and T. A. Miller. 2006. Insect symbiosis, vol. 2. CRC Press, New York, NY.
-
- Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, NY.
-
- Chang, K. P. 1974. Effects of elevated temperature on the mycetome and symbiotes of the bed bug Cimex lectularius (Heteroptera). J. Invertebr. Pathol. 23:333-340. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

