Differential effects of temperature on natural transformation to erythromycin and nalidixic acid resistance in Campylobacter coli
- PMID: 18708520
- PMCID: PMC2565985
- DOI: 10.1128/AEM.01075-08
Differential effects of temperature on natural transformation to erythromycin and nalidixic acid resistance in Campylobacter coli
Abstract
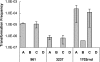
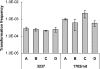
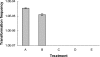
Campylobacter jejuni and Campylobacter coli are naturally competent, but limited information exists on the impact of environmental conditions on transformation. In this study, we investigated the impact of temperature and microaerobic versus aerobic atmosphere on transformation of C. coli to erythromycin and nalidixic acid resistance. Frequency of transformation was not significantly different between microaerobic (5 to 10% CO(2)) and aerobic conditions. However, C. coli was transformed to erythromycin resistance at a significantly higher frequency at 42 degrees C than at 25 degrees C (P < 0.05), and few or no transformants were obtained at 25 degrees C. In contrast, transformation to nalidixic acid resistance was highly efficient at both 42 degrees C and 25 degrees C and was similar or, at the most, fourfold higher at 42 degrees C than at 25 degrees C. DNase I treatment experiments suggested that steps both prior and subsequent to internalization of DNA were influenced by temperature in the case of transformation of C. coli to erythromycin resistance. However, the moderately increased (fourfold) frequency of transformation to nalidixic acid resistance at 42 degrees C compared to that at 25 degrees C was exclusively associated with steps prior to DNA internalization. These findings suggest that transformation to erythromycin resistance may be significantly more frequent in the gastrointestinal tract of hosts such as poultry (at 42 degrees C) than in other habitats characterized by lower temperatures, whereas transformation to nalidixic acid resistance may be highly efficient both within and outside the animal hosts.
Figures
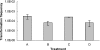
Similar articles
-
The Current State of Macrolide Resistance in Campylobacter spp.: Trends and Impacts of Resistance Mechanisms.Appl Environ Microbiol. 2017 May 31;83(12):e00416-17. doi: 10.1128/AEM.00416-17. Print 2017 Jun 15. Appl Environ Microbiol. 2017. PMID: 28411226 Free PMC article. Review.
-
Natural transformation-mediated transfer of erythromycin resistance in Campylobacter coli strains from turkeys and swine.Appl Environ Microbiol. 2006 Feb;72(2):1316-21. doi: 10.1128/AEM.72.2.1316-1321.2006. Appl Environ Microbiol. 2006. PMID: 16461682 Free PMC article.
-
Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype.Antimicrob Agents Chemother. 2005 Jul;49(7):2753-9. doi: 10.1128/AAC.49.7.2753-2759.2005. Antimicrob Agents Chemother. 2005. PMID: 15980346 Free PMC article.
-
Prevalence and antimicrobial resistance of Campylobacter in US dairy cattle.J Appl Microbiol. 2007 Jun;102(6):1570-7. doi: 10.1111/j.1365-2672.2006.03189.x. J Appl Microbiol. 2007. PMID: 17578422
-
Macrolide resistance in Campylobacter jejuni and Campylobacter coli.J Antimicrob Chemother. 2006 Aug;58(2):243-55. doi: 10.1093/jac/dkl210. Epub 2006 May 30. J Antimicrob Chemother. 2006. PMID: 16735431 Review.
Cited by
-
The Current State of Macrolide Resistance in Campylobacter spp.: Trends and Impacts of Resistance Mechanisms.Appl Environ Microbiol. 2017 May 31;83(12):e00416-17. doi: 10.1128/AEM.00416-17. Print 2017 Jun 15. Appl Environ Microbiol. 2017. PMID: 28411226 Free PMC article. Review.
-
Steady at the wheel: conservative sex and the benefits of bacterial transformation.Philos Trans R Soc Lond B Biol Sci. 2016 Oct 19;371(1706):20150528. doi: 10.1098/rstb.2015.0528. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27619692 Free PMC article. Review.
-
Natural transformation of Campylobacter jejuni occurs beyond limits of growth.PLoS One. 2012;7(9):e45467. doi: 10.1371/journal.pone.0045467. Epub 2012 Sep 26. PLoS One. 2012. PMID: 23049803 Free PMC article.
-
Differences in the Propensity of Different Antimicrobial Resistance Determinants to Be Disseminated via Transformation in Campylobacter jejuni and Campylobacter coli.Microorganisms. 2022 Jun 10;10(6):1194. doi: 10.3390/microorganisms10061194. Microorganisms. 2022. PMID: 35744712 Free PMC article.
-
"Take It or Leave It"-Factors Regulating Competence Development and DNA Uptake in Campylobacter jejuni.Int J Mol Sci. 2021 Sep 21;22(18):10169. doi: 10.3390/ijms221810169. Int J Mol Sci. 2021. PMID: 34576332 Free PMC article.
References
-
- Alm, R. A., P. Guerry, and T. J. Trust. 1993. Significance of duplicated flagellin genes in Campylobacter. J. Mol. Biol. 230:359-363. - PubMed
-
- Avrain, L., F. Humbert, R. L'Hospitalier, P. Sanders, C. Vernozy-Rozand, and I. Kempf. 2003. Antimicrobial resistance in Campylobacter from broilers: association with production type and antimicrobial use. Vet. Microbiol. 96:267-276. - PubMed
-
- Carattoli, A., A. M. Dionisi, and I. Luzzi. 2002. Use of a LightCycler gyrA mutation assay for identification of ciprofloxacin-resistant Campylobacter coli. FEMS Microbiol. Lett. 214:87-93. - PubMed
-
- de Boer, P., J. A. Wagenaar, R. P. Achterberg, J. P. M. van Putten, L. M. Schouls, and B. Duim. 2002. Generation of Campylobacter jejuni genetic diversity in vivo. Mol. Microbiol. 44:351-359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

