Plant hormone receptors: new perceptions
- PMID: 18708574
- PMCID: PMC2735353
- DOI: 10.1101/gad.1693208
Plant hormone receptors: new perceptions
Abstract
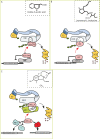
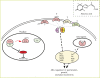
Plant growth and development require the integration of a variety of environmental and endogenous signals that, together with the intrinsic genetic program, determine plant form. Central to this process are several growth regulators known as plant hormones or phytohormones. Despite decades of study, only recently have receptors for several of these hormones been identified, revealing novel mechanisms for perceiving chemical signals and providing plant biologists with a much clearer picture of hormonal control of growth and development.
Figures
Similar articles
-
Plant hormone receptors: perception is everything.Genes Dev. 2006 Aug 1;20(15):1998-2008. doi: 10.1101/gad.1432806. Genes Dev. 2006. PMID: 16882977 Review.
-
Signs of change: hormone receptors that regulate plant development.Development. 2006 May;133(10):1857-69. doi: 10.1242/dev.02359. Development. 2006. PMID: 16651539 Review.
-
Recent advances in the study of mechanisms of action of phytohormones.Biochemistry (Mosc). 2004 Mar;69(3):233-47. doi: 10.1023/b:biry.0000022053.73461.cd. Biochemistry (Mosc). 2004. PMID: 15061689 Review.
-
Ligand-receptor interactions in plant hormone signaling.Plant J. 2021 Jan;105(2):290-306. doi: 10.1111/tpj.15115. Epub 2021 Jan 8. Plant J. 2021. PMID: 33278046 Review.
-
Receptors and signalling components of plant hormones.J Recept Signal Transduct Res. 1999 Jan-Jul;19(1-4):41-58. doi: 10.3109/10799899909036636. J Recept Signal Transduct Res. 1999. PMID: 10071749 Review.
Cited by
-
Structural insights into AtABCG25, an angiosperm-specific abscisic acid exporter.Plant Commun. 2024 Jan 8;5(1):100776. doi: 10.1016/j.xplc.2023.100776. Epub 2023 Dec 3. Plant Commun. 2024. PMID: 38050355 Free PMC article.
-
Isolation and characterization of gene encoding G protein α subunit protein responsive to plant hormones and abiotic stresses in Brassica napus.Mol Biol Rep. 2010 Dec;37(8):3957-65. doi: 10.1007/s11033-010-0054-x. Epub 2010 Mar 18. Mol Biol Rep. 2010. PMID: 20238175
-
Gene expression analysis and halting of ethylene receptors signaling pinpoint ethylene as a positive regulator of direct somatic embryogenesis in Coffea arabica.Planta. 2025 Aug 8;262(3):74. doi: 10.1007/s00425-025-04798-8. Planta. 2025. PMID: 40779278
-
Jasmonate signalling in Arabidopsis involves SGT1b-HSP70-HSP90 chaperone complexes.Nat Plants. 2015;1:15049. doi: 10.1038/nplants.2015.49. Epub 2015 Apr 27. Nat Plants. 2015. PMID: 27054042 Free PMC article.
-
A maize phytochrome-interacting factor 3 improves drought and salt stress tolerance in rice.Plant Mol Biol. 2015 Mar;87(4-5):413-28. doi: 10.1007/s11103-015-0288-z. Epub 2015 Jan 31. Plant Mol Biol. 2015. PMID: 25636202
References
-
- Benavente L.M., Alonso J.M. Molecular mechanisms of ethylene signaling in Arabidopsis. Mol. Biosyst. 2006;2:165–173. - PubMed
-
- Chini A., Fonseca S., Fernandez G., Adie B., Chico J.M., Lorenzo O., Garcia-Casado G., Lopez-Vidriero I., Lozano F.M., Ponce M.R., et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. - PubMed
-
- Chow B., McCourt P. Plant hormone receptors: Perception is everything. Genes & Dev. 2006;20:1998–2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
