Tumorigenic activity and therapeutic inhibition of Rheb GTPase
- PMID: 18708578
- PMCID: PMC2518821
- DOI: 10.1101/gad.1690808
Tumorigenic activity and therapeutic inhibition of Rheb GTPase
Abstract
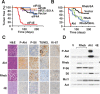
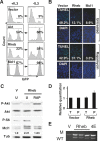
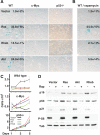
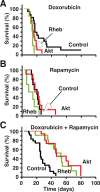
The AKT-mTOR pathway harbors several known and putative oncogenes and tumor suppressors. In a phenotypic screen for lymphomagenesis, we tested candidate genes acting upstream of and downstream from mTOR in vivo. We find that Rheb, a proximal activator of mTORC1, can produce rapid development of aggressive and drug-resistant lymphomas. Rheb causes mTORC1-dependent effects on apoptosis, senescence, and treatment responses that resemble those of Akt. Moreover, Rheb activity toward mTORC1 requires farnesylation and is readily blocked by a pharmacological inhibitor of farnesyltransferase (FTI). In Pten-deficient tumor cells, inhibition of Rheb by FTI is responsible for the drug's anti-tumor effects, such that a farnesylation-independent mutant of Rheb renders these tumors resistant to FTI therapy. Notably, RHEB is highly expressed in some human lymphomas, resulting in mTORC1 activation and increased sensitivity to rapamycin and FTI. Downstream from mTOR, we examined translation initiation factors that have been implicated in transformation in vitro. Of these, only eIF4E was able to enhance lymphomagenesis in vivo. In summary, the Rheb GTPase is an oncogenic activity upstream of mTORC1 and eIF4E and a direct therapeutic target of farnesyltransferase inhibitors in cancer.
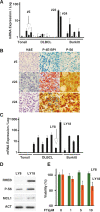
Figures

References
-
- Avdulov S., Li S., Michalek V., Burrichter D., Peterson M., Perlman D.M., Manivel J.C., Sonenberg N., Yee D., Bitterman P.B., et al. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell. 2004;5:553–563. - PubMed
-
- Basso A.D., Mirza A., Liu G., Long B.J., Bishop W.R., Kirschmeier P. The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J. Biol. Chem. 2005;280:31101–31108. - PubMed
-
- Braun T., Fenaux P. Farnesyltransferase inhibitors and their potential role in therapy for myelodysplastic syndromes and acute myeloid leukaemia. Br. J. Haematol. 2008;141:576–586. - PubMed
-
- Buerger C., DeVries B., Stambolic V. Localization of Rheb to the endomembrane is critical for its signaling function. Biochem. Biophys. Res. Commun. 2006;344:869–880. - PubMed
-
- Burnett A.K., Kell J. Tipifarnib in acute myeloid leukemia. Drugs Today (Barc) 2007;43:795–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
