NFAT regulates induction of COX-2 and apoptosis of keratinocytes in response to ultraviolet radiation exposure
- PMID: 18708588
- PMCID: PMC2671982
- DOI: 10.1096/fj.08-113076
NFAT regulates induction of COX-2 and apoptosis of keratinocytes in response to ultraviolet radiation exposure
Abstract
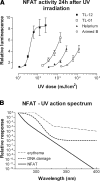
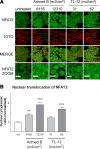
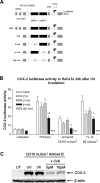
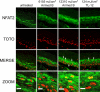
The nuclear factor of activated T cells (NFAT) transcription factors are regulated by calcium/calcineurin signals and play important roles in T cells, muscle, bone, and neural tissue. NFAT is expressed in the epidermis, and although recent data suggest that NFAT is involved in the skin's responses to ultraviolet radiation (UVR), the wavelengths of radiation that activate NFAT and the biological function of UV-activated NFAT remain undefined. We demonstrate that NFAT transcriptional activity is preferentially induced by UVB wavelengths in keratinocytes. The derived action spectrum for NFAT activation indicates that NFAT transcriptional activity is inversely associated with wavelength. UVR also evoked NFAT2 nuclear translocation in a parallel wavelength-dependent fashion and both transcriptional activation and nuclear translocation were inhibited by the calcineurin inhibitor cyclosporin A. UVR also evoked NFAT2 nuclear translocation in three-dimensional skin equivalents. Evidence suggests that COX-2 contributes to UV-induced carcinogenesis. Inhibiting UV-induced NFAT activation in keratinocytes, reduced COX-2 protein induction, and increased UV-induced apoptosis. COX-2 luciferase reporters lacking functional NFAT binding sites were less responsive to UVR, highlighting that NFAT is required for UV-induced COX-2 induction. Taken together, these data suggest that the proinflammatory, antiapoptotic, and procarcinogenic functions of UV-activated COX-2 may be mediated, in part, by upstream NFAT signaling.
Figures
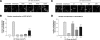
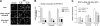
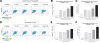
Similar articles
-
Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratinocytes by cyclosporin A.J Invest Dermatol. 2002 May;118(5):779-88. doi: 10.1046/j.1523-1747.2002.01709.x. J Invest Dermatol. 2002. PMID: 11982754
-
NFAT signalling is a novel target of oncogenic BRAF in metastatic melanoma.Br J Cancer. 2009 Oct 20;101(8):1448-55. doi: 10.1038/sj.bjc.6605277. Epub 2009 Sep 1. Br J Cancer. 2009. PMID: 19724275 Free PMC article.
-
Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors.Mol Carcinog. 2007 Apr;46(4):303-14. doi: 10.1002/mc.20281. Mol Carcinog. 2007. PMID: 17186551
-
Calcineurin/NFAT-dependent regulation of 230-kDa bullous pemphigoid antigen (BPAG1) gene expression in normal human epidermal keratinocytes.J Dermatol Sci. 2008 Jul;51(1):45-51. doi: 10.1016/j.jdermsci.2008.01.006. Epub 2008 Mar 18. J Dermatol Sci. 2008. PMID: 18353617
-
Calcium-NFAT transcriptional signalling in T cell activation and T cell exhaustion.Cell Calcium. 2017 May;63:66-69. doi: 10.1016/j.ceca.2017.01.014. Epub 2017 Jan 28. Cell Calcium. 2017. PMID: 28153342 Free PMC article. Review.
Cited by
-
Comparison of low and high dose ionising radiation using topological analysis of gene coexpression networks.BMC Genomics. 2012 May 17;13:190. doi: 10.1186/1471-2164-13-190. BMC Genomics. 2012. PMID: 22594378 Free PMC article.
-
Upregulation of nuclear factor of activated T-cells by nerve injury contributes to development of neuropathic pain.J Pharmacol Exp Ther. 2013 Apr;345(1):161-8. doi: 10.1124/jpet.112.202192. Epub 2013 Feb 5. J Pharmacol Exp Ther. 2013. PMID: 23386250 Free PMC article.
-
Cyclooxygenase-2 (COX-2) mediates arsenite inhibition of UVB-induced cellular apoptosis in mouse epidermal Cl41 cells.Curr Cancer Drug Targets. 2012 Jul;12(6):607-16. doi: 10.2174/156800912801784802. Curr Cancer Drug Targets. 2012. PMID: 22463588 Free PMC article.
-
Pimecrolimus interferes the therapeutic efficacy of human mesenchymal stem cells in atopic dermatitis by regulating NFAT-COX2 signaling.Stem Cell Res Ther. 2021 Aug 28;12(1):482. doi: 10.1186/s13287-021-02547-8. Stem Cell Res Ther. 2021. PMID: 34454603 Free PMC article.
-
Progesterone inhibition of voltage-gated calcium channels is a potential neuroprotective mechanism against excitotoxicity.Steroids. 2011 Aug;76(9):845-55. doi: 10.1016/j.steroids.2011.02.013. Epub 2011 Mar 1. Steroids. 2011. PMID: 21371490 Free PMC article.
References
-
- Shaw J P, Utz P J, Durand D B, Toole J J, Emmel E A, Crabtree G R. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. - PubMed
-
- Kiani A, Rao A, Aramburu J. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity. 2000;12:359–372. - PubMed
-
- Hill-Eubanks D C, Gomez M F, Stevenson A S, Nelson M T. NFAT regulation in smooth muscle. Trends Cardiovasc Med. 2003;13:56–62. - PubMed
-
- Schulz R A, Yutzey K E. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol. 2004;266:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

