Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants
- PMID: 18708632
- PMCID: PMC2584876
- DOI: 10.1152/ajplung.90240.2008
Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants
Abstract
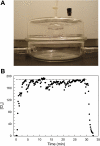
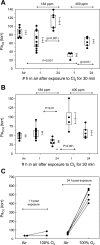
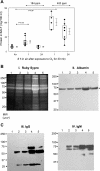
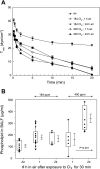
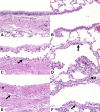
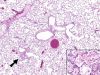
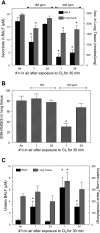
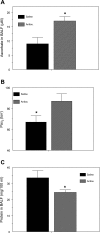
Chlorine (Cl(2)) is a highly reactive oxidant gas used extensively in a number of industrial processes. Exposure to high concentrations of Cl(2) results in acute lung injury that may either resolve spontaneously or progress to acute respiratory failure. Presently, the pathophysiological sequelae associated with Cl(2)-induced acute lung injury in conscious animals, as well as the cellular and biochemical mechanisms involved, have not been elucidated. We exposed conscious Sprague-Dawley rats to Cl(2) gas (184 or 400 ppm) for 30 min in environmental chambers and then returned them to room air. At 1 h after exposure, rats showed evidence of arterial hypoxemia, respiratory acidosis, increased levels of albumin, IgG, and IgM in bronchoalveolar lavage fluid (BALF), increased BALF surfactant surface tension, and significant histological injury to airway and alveolar epithelia. These changes were more pronounced in the 400-ppm-exposed rats. Concomitant decreases of ascorbate (AA) and reduced glutathione (GSH) were also detected in both BALF and lung tissues. In contrast, heart tissue AA and GSH content remained unchanged. These abnormalities persisted 24 h after exposure in rats exposed to 400 ppm Cl(2). Rats injected systemically with a mixture of AA, deferoxamine, and N-acetyl-L-cysteine before exposure to 184 ppm Cl(2) had normal levels of AA, lower levels of BALF albumin and normal arterial Po(2) and Pco(2) values. These findings suggest that Cl(2) inhalation damages both airway and alveolar epithelial tissues and that resulting effects were ameliorated by prophylactic administration of low-molecular-weight antioxidants.
Figures
References
-
- Ballinger CA, Cueto R, Squadrito G, Coffin JF, Velsor LW, Pryor WA, Postlethwait EM. Antioxidant-mediated augmentation of ozone-induced membrane oxidation. Free Radic Biol Med 38: 515–526, 2005. - PubMed
-
- Batchinsky AI, Martini DK, Jordan BS, Dick EJ, Fudge J, Baird CA, Hardin DE, Cancio LC. Acute respiratory distress syndrome secondary to inhalation of chlorine gas in sheep. J Trauma 60: 944–956, 2006. - PubMed
-
- Bell DG Management of acute respiratory distress syndrome (ARDS) following chlorine exposure (Abstract). Am J Respir Crit Care Med 176: A314, 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

