Peptide-targeted polyglutamic acid doxorubicin conjugates for the treatment of alpha(v)beta(6)-positive cancers
- PMID: 18710273
- PMCID: PMC4053474
- DOI: 10.1021/bc800154f
Peptide-targeted polyglutamic acid doxorubicin conjugates for the treatment of alpha(v)beta(6)-positive cancers
Abstract
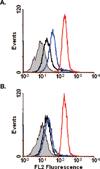
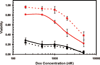
Most chemotherapeutics exert their effects on tumor cells as well as their healthy counterparts, resulting in dose limiting side effects. Cell-specific delivery of therapeutics can increase the therapeutic window for treatment by maintaining the therapeutic efficacy while decreasing the untoward side effects. We have previously identified a peptide, named H2009.1, which binds to the integrin alpha(v)beta(6). Here, we report the synthesis of a peptide targeted polyglutamic acid polymer in which the high affinity alpha(v)beta(6)-specific tetrameric H2009.1 peptide is incorporated via a thioether at the N-terminus of a 15 amino acid polymer of glutamic acid. Doxorubicin is incorporated into the polymer via an acid-labile hydrazone bond. Payloads of four doxorubicin molecules per targeting agent are achieved. The drug is released at pH 4.0 and 5.6 but the conjugate is stable at pH 7.0. The conjugate is selectively internalized into alpha(v)beta(6) positive cells as witnessed by flow cytometric analysis and fluorescent microscopy. Cellular uptake is mediated by the H2009.1 peptide, as no internalization of the doxorubicin-PG polymer is observed when it is conjugated to a scrambled sequence control peptide. Importantly, the conjugate is more cytotoxic toward a targeted cell than a cell line that does not express the integrin.
Figures



Similar articles
-
From phage display to nanoparticle delivery: functionalizing liposomes with multivalent peptides improves targeting to a cancer biomarker.Bioconjug Chem. 2013 Jan 16;24(1):85-96. doi: 10.1021/bc300498d. Epub 2013 Jan 7. Bioconjug Chem. 2013. PMID: 23186007 Free PMC article.
-
Water-soluble polymers for targeted drug delivery to human squamous carcinoma of head and neck.J Drug Target. 2005 Apr;13(3):189-97. doi: 10.1080/10611860500065187. J Drug Target. 2005. PMID: 16036307
-
Polymer conjugates of doxorubicin bound through an amide and hydrazone bond: Impact of the carrier structure onto synergistic action in the treatment of solid tumours.Eur J Pharm Sci. 2014 Jul 16;58:1-12. doi: 10.1016/j.ejps.2014.02.016. Epub 2014 Mar 13. Eur J Pharm Sci. 2014. PMID: 24632485
-
Targeting doxorubicin to epidermal growth factor receptors by site-specific conjugation of C225 to poly(L-glutamic acid) through a polyethylene glycol spacer.Pharm Res. 2003 May;20(5):826-32. doi: 10.1023/a:1023454107190. Pharm Res. 2003. PMID: 12751641
-
Novel peptide conjugates for tumor-specific chemotherapy.J Med Chem. 2001 Apr 26;44(9):1341-8. doi: 10.1021/jm001065f. J Med Chem. 2001. PMID: 11311056
Cited by
-
Tumor-targeting peptides from combinatorial libraries.Adv Drug Deliv Rev. 2017 Feb;110-111:13-37. doi: 10.1016/j.addr.2016.05.009. Epub 2016 May 19. Adv Drug Deliv Rev. 2017. PMID: 27210583 Free PMC article. Review.
-
Generation of new peptide-Fc fusion proteins that mediate antibody-dependent cellular cytotoxicity against different types of cancer cells.Mol Ther Methods Clin Dev. 2015 Nov 4;2:15043. doi: 10.1038/mtm.2015.43. eCollection 2015. Mol Ther Methods Clin Dev. 2015. PMID: 26605373 Free PMC article.
-
ASGR1 and Its Enigmatic Relative, CLEC10A.Int J Mol Sci. 2020 Jul 8;21(14):4818. doi: 10.3390/ijms21144818. Int J Mol Sci. 2020. PMID: 32650396 Free PMC article. Review.
-
Possible contribution of beta-glycosidases and caspases in the cytotoxicity of novel glycoconjugates in colon cancer cells.Invest New Drugs. 2010 Jun;28(3):306-17. doi: 10.1007/s10637-009-9248-2. Epub 2009 May 5. Invest New Drugs. 2010. PMID: 19415182
-
Combinatorial peptide libraries: mining for cell-binding peptides.Chem Rev. 2014 Jan 22;114(2):1020-81. doi: 10.1021/cr400166n. Epub 2013 Dec 3. Chem Rev. 2014. PMID: 24299061 Free PMC article. Review. No abstract available.
References
-
- Oyama T, Sykes KF, Samli KN, Minna JD, Johnston SA, Brown KC. Isolation of lung tumor specific peptides from a random peptide library: generation of diagnostic and cell-targeting reagents. Cancer Lett. 2003;202:219–230. - PubMed
-
- Zhou X, Chang Y, Oyama T, McGuire MJ, Brown KC. Cell-specific delivery of a chemotherapeutic to lung cancer cells. J. Am. Chem. Soc. 2004;129:15656–15657. - PubMed
-
- Elayadi AN, Samli KN, Prudkin L, Liu YH, Bian A, Xie XJ, Roth JA, Wistuba II, McGuire MJ, Brown KC. A peptide selected by biopanning identifies the integrin αvβ6 as a prognostic biomarker for non-small cell lung cancer. Cancer Res. 2007;67:5889–5895. - PubMed
-
- Li C. Poly(l-glutamic acid)-anticancer drug conjugates. Adv. Drug Delivery Rev. 2002;54:695–713. - PubMed
-
- Vega J, Ke S, Fan Z, Wallace S, Charsangavej C, Li C. Targeting doxorubicin to epidermal growth factor receptors by site-specific conjugation of C225 to poly(l-glutamic acid) through a polyethylene glycol spacer. Pharm. Res. 2003;20:826–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

