Critical role of acrolein in secondary injury following ex vivo spinal cord trauma
- PMID: 18710419
- PMCID: PMC2671023
- DOI: 10.1111/j.1471-4159.2008.05622.x
Critical role of acrolein in secondary injury following ex vivo spinal cord trauma
Abstract
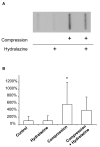
The pathophysiology of spinal cord injury (SCI) is characterized by the initial, primary injury followed by secondary injury processes in which oxidative stress is a critical component. Secondary injury processes not only exacerbate pathology at the site of primary injury, but also result in spreading of injuries to the adjacent, otherwise healthy tissue. The lipid peroxidation byproduct acrolein has been implicated as one potential mediator of secondary injury. To further and rigorously elucidate the role of acrolein in secondary injury, a unique ex vivo model is utilized to isolate the detrimental effects of mechanical injury from toxins such as acrolein that are produced endogenously following SCI. We demonstrate that (i) acrolein-Lys adducts are capable of diffusing from compressed tissue to adjacent, otherwise uninjured tissue; (ii) secondary injury by itself produces significant membrane damage and increased superoxide production; and (iii) these injuries are significantly attenuated by the acrolein scavenger hydralazine. Furthermore, hydralazine treatment results in significantly less membrane damage 2 h following compression injury, but not immediately after. These findings support our hypothesis that, following SCI, acrolein is increased to pathologic concentrations, contributes significantly to secondary injury, and thus represents a novel target for scavenging to promote improved recovery.
Figures






References
-
- Adams JD, Jr., Klaidman LK. Acrolein-induced oxygen radical formation. Free Radic. Biol. Med. 1993;15:187–193. - PubMed
-
- Braughler JM, Hall ED. Central nervous system trauma and stroke I. Biochemical considerations for oxygen radical formation and lipid peroxidation. Free Radic. Biol. Med. 1989;6:289–301. - PubMed
-
- Burcham PC, Pyke SM. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol. Pharmacol. 2006;69:1056–1065. - PubMed
-
- Burcham PC, Kerr PG, Fontaine F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000;5:47–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

