Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation
- PMID: 18710878
- PMCID: PMC2533423
- DOI: 10.1093/cvr/cvn184
Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation
Abstract
Aims: Mitochondrial dysfunction is a major factor in heart failure (HF). A pronounced variability of mitochondrial electron transport chain (ETC) defects is reported to occur in severe acquired cardiomyopathies without a consistent trend for depressed activity or expression. The aim of this study was to define the defect in the integrative function of cardiac mitochondria in coronary microembolization-induced HF.
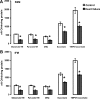
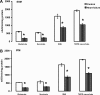
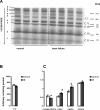
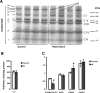
Methods and results: Studies were performed in the canine coronary microembolization-induced HF model of moderate severity. Oxidative phosphorylation was assessed as the integrative function of mitochondria, using a comprehensive variety of substrates in order to investigate mitochondrial membrane transport, dehydrogenase activity and electron-transport coupled to ATP synthesis. The supramolecular organization of the mitochondrial ETC also was investigated by native gel electrophoresis. We found a dramatic decrease in ADP-stimulated respiration that was not relieved by an uncoupler. Moreover, the ADP/O ratio was normal, indicating no defect in the phosphorylation apparatus. The data point to a defect in oxidative phosphorylation within the ETC. However, the individual activities of ETC complexes were normal. The amount of the supercomplex consisting of complex I/complex III dimer/complex IV, the major form of respirasome considered essential for oxidative phosphorylation, was decreased.
Conclusions: We propose that the mitochondrial defect lies in the supermolecular assembly rather than in the individual components of the ETC.
Figures
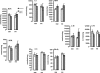

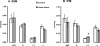
Comment in
-
Lack of functional assembly in mitochondrial supercomplexes: a new insight into impaired mitochondrial function?Cardiovasc Res. 2008 Oct 1;80(1):3-4. doi: 10.1093/cvr/cvn213. Epub 2008 Aug 7. Cardiovasc Res. 2008. PMID: 18687704 No abstract available.
References
-
- DiMauro S, Schon EA. Mitochondrial DNA mutations in human disease. Am J Med Genet. 2001;106:18–26. - PubMed
-
- Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–2367. - PubMed
-
- Dorner A, Giessen S, Gaub R, Grosse Siestrup H, Schwimmbeck PL, Hetzer R, et al. An isoform shift in the cardiac adenine nucleotide translocase expression alters the kinetic properties of the carrier in dilated cardiomyopathy. Eur J Heart Fail. 2006;8:81–89. - PubMed
-
- Jarreta D, Orus J, Barrientos A, Miro O, Roig E, Heras M, et al. Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovasc Res. 2000;45:860–865. - PubMed
-
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

