Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation
- PMID: 18710921
- PMCID: PMC2518713
- DOI: 10.1083/jcb.200801062
Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation
Abstract
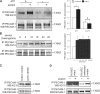
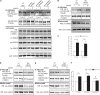
Mechanosensing followed by mechanoresponses by cells is well established, but the mechanisms by which mechanical force is converted into biochemical events are poorly understood. Vascular endothelial cells (ECs) exhibit flow- and stretch-dependent responses and are widely used as a model for studying mechanotransduction in mammalian cells. Platelet EC adhesion molecule 1 (PECAM-1) is tyrosine phosphorylated when ECs are exposed to flow or when PECAM-1 is directly pulled, suggesting that it is a mechanochemical converter. We show that PECAM-1 phosphorylation occurs when detergent-extracted EC monolayers are stretched, indicating that this phosphorylation is mechanically triggered and does not require the intact plasma membrane and soluble cytoplasmic components. Using kinase inhibitors and small interfering RNAs, we identify Fyn as the PECAM-1 kinase associated with the model. We further show that stretch- and flow-induced PECAM-1 phosphorylation in intact ECs is abolished when Fyn expression is down-regulated. We suggest that PECAM-1 and Fyn are essential components of a PECAM-1-based mechanosensory complex in ECs.
Figures



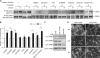

References
-
- Akiyama, T., and H. Ogawara. 1991. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 201:362–370. - PubMed
-
- Bagi, Z., J.A. Frangos, J.C. Yeh, C.R. White, G. Kaley, and A. Koller. 2005. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler. Thromb. Vasc. Biol. 25:1590–1595. - PubMed
-
- Cao, M.Y., M. Huber, N. Beauchemin, J. Famiglietti, S.M. Albelda, and A. Veillette. 1998. Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J. Biol. Chem. 273:15765–15772. - PubMed
-
- Chau, C.H., K.Y. Chen, H.T. Deng, K.J. Kim, K. Hosoya, T. Terasaki, H.M. Shih, and D.K. Ann. 2002. Coordinating Etk/Bmx activation and VEGF upregulation to promote cell survival and proliferation. Oncogene. 21:8817–8829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

