Regulation of the endosomal SNARE protein syntaxin 7 by colony-stimulating factor 1 in macrophages
- PMID: 18710945
- PMCID: PMC2577439
- DOI: 10.1128/MCB.00220-08
Regulation of the endosomal SNARE protein syntaxin 7 by colony-stimulating factor 1 in macrophages
Abstract
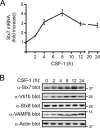
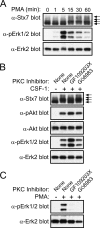
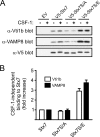
Colony-stimulating factor 1 (CSF-1) is the main growth factor controlling the development of macrophages from myeloid progenitor cells. However, CSF-1 also regulates some of the key effector functions of macrophages (e.g., phagocytosis and cytokine secretion). The endosomal SNARE protein syntaxin 7 (Stx7) regulates vesicle trafficking events involved in phagocytosis and cytokine secretion. Therefore, we investigated the ability of CSF-1 to regulate Stx7. CSF-1 upregulated Stx7 expression in primary mouse macrophages; it also upregulated expression of its SNARE partners Vti1b and VAMP8 but not Stx8. Additionally, CSF-1 induced the rapid serine phosphorylation of Stx7 and enhanced its binding to Vti1b, Stx8, and VAMP8. Bioinformatics analysis and results from experiments with kinase inhibitors suggested the CSF-1-induced phosphorylation of Stx7 was mediated by protein kinase C and Akt in response to phosphatidylinositol 3-kinase activation. Based on mutagenesis studies, CSF-1 appeared to increase the binding of Stx7 to its SNARE partners by inducing the phosphorylation of serine residues in the Habc domain and/or "linker" region of Stx7. Thus, CSF-1 is a key regulator of Stx7 expression and function in macrophages. Furthermore, the effects of CSF-1 on Stx7 may provide a mechanism for the regulation of macrophage effector functions by CSF-1.
Figures




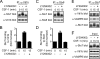


Similar articles
-
Syntaxin 11 binds Vti1b and regulates late endosome to lysosome fusion in macrophages.Traffic. 2011 Jun;12(6):762-73. doi: 10.1111/j.1600-0854.2011.01189.x. Epub 2011 Apr 8. Traffic. 2011. PMID: 21388490
-
Salmonella Typhimurium Manipulates Syntaxin 7 to Navigate Endo-Lysosomal Trafficking in Host Cells.Traffic. 2025 Apr-Jun;26(4-6):e70010. doi: 10.1111/tra.70010. Traffic. 2025. PMID: 40444290
-
The trans-SNARE complex VAMP4/Stx6/Stx7/Vti1b is a key regulator of Golgi to late endosome MT1-MMP transport in macrophages.Traffic. 2021 Nov;22(11):368-376. doi: 10.1111/tra.12813. Epub 2021 Sep 13. Traffic. 2021. PMID: 34476885
-
Biology and action of colony--stimulating factor-1.Mol Reprod Dev. 1997 Jan;46(1):4-10. doi: 10.1002/(SICI)1098-2795(199701)46:1<4::AID-MRD2>3.0.CO;2-V. Mol Reprod Dev. 1997. PMID: 8981357 Review.
-
CSF-1 signal transduction.J Leukoc Biol. 1997 Aug;62(2):145-55. doi: 10.1002/jlb.62.2.145. J Leukoc Biol. 1997. PMID: 9261328 Review.
Cited by
-
Peripheral blood gene expression profiles in COPD subjects.J Clin Bioinforma. 2011 Apr 24;1(1):12. doi: 10.1186/2043-9113-1-12. J Clin Bioinforma. 2011. PMID: 21884629 Free PMC article.
-
GIFT: Guided and Interpretable Factorization for Tensors with an application to large-scale multi-platform cancer analysis.Bioinformatics. 2018 Dec 15;34(24):4151-4158. doi: 10.1093/bioinformatics/bty490. Bioinformatics. 2018. PMID: 29931238 Free PMC article.
-
Phosphoproteomics reveals that the hVPS34 regulated SGK3 kinase specifically phosphorylates endosomal proteins including Syntaxin-7, Syntaxin-12, RFIP4 and WDR44.Biochem J. 2019 Oct 30;476(20):3081-3107. doi: 10.1042/BCJ20190608. Biochem J. 2019. PMID: 31665227 Free PMC article.
-
Mfsd2a (Major Facilitator Superfamily Domain Containing 2a) Attenuates Intracerebral Hemorrhage-Induced Blood-Brain Barrier Disruption by Inhibiting Vesicular Transcytosis.J Am Heart Assoc. 2017 Jul 19;6(7):e005811. doi: 10.1161/JAHA.117.005811. J Am Heart Assoc. 2017. PMID: 28724654 Free PMC article.
-
Sensing of immature particles produced by dengue virus infected cells induces an antiviral response by plasmacytoid dendritic cells.PLoS Pathog. 2014 Oct 23;10(10):e1004434. doi: 10.1371/journal.ppat.1004434. eCollection 2014 Oct. PLoS Pathog. 2014. PMID: 25340500 Free PMC article.
References
-
- Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17593-623. - PubMed
-
- Antonin, W., I. Dulubova, D. Arac, S. Pabst, J. Plitzner, J. Rizo, and R. Jahn. 2002. The N-terminal domains of syntaxin 7 and vti1b form three-helix bundles that differ in their ability to regulate SNARE complex assembly. J. Biol. Chem. 27736449-36456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
