Breast tumor xenografts: diffusion-weighted MR imaging to assess early therapy with novel apoptosis-inducing anti-DR5 antibody
- PMID: 18710978
- PMCID: PMC2657851
- DOI: 10.1148/radiol.2483071740
Breast tumor xenografts: diffusion-weighted MR imaging to assess early therapy with novel apoptosis-inducing anti-DR5 antibody
Abstract
Purpose: To measure the early therapeutic response to a novel apoptosis-inducing antibody, TRA-8, by using diffusion-weighted magnetic resonance (MR) imaging in a mouse breast cancer model.
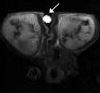
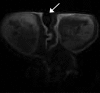
Materials and methods: Animal experiments had institutional animal care and use committee approval. Four groups of nude mice bearing luciferase-positive breast tumors (four to five mice with eight to 10 tumors per group) were injected intravenously with 0 mg (group 1), 0.025 mg (group 2), 0.100 mg (group 3), or 0.200 mg (group 4) of TRA-8 on days 0 and 3. Diffusion-weighted imaging, anatomic MR imaging, and bioluminescence imaging were performed on days 0, 3, and 6 before dosing. Averaged apparent diffusion coefficients (ADCs) for both whole tumor volume and a 1-mm peripheral tumor shell were calculated and were compared with tumor volume and living tumor cell changes. After imaging at day 6, proliferating and apoptotic cell densities were measured with Ki67 and terminal deoxynucleotidyl transferase mediated dUTP nick end labeling, or TUNEL, staining, respectively, and were compared with cleaved caspase-3 density.
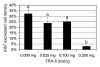
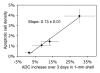
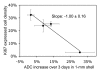
Results: The ADC increase at day 3 was dependent on TRA-8 dose level, averaging 6% +/- 3 (standard error of mean), 19% +/- 4, 14% +/- 4, and 34% +/- 7 in the whole tumor volume and 1% +/- 2, 9% +/- 5, 13% +/- 5, and 30% +/- 8 in the outer 1-mm tumor shell only for groups 1, 2, 3, and 4, respectively. The ADC increase in group 4 was significantly higher (P = .0008 and P = .0189 for whole tumor volume and peripheral region, respectively) than that in group 1 on day 3, whereas tumor size did not significantly differ. At day 3, the dose-dependent ADC increases were linearly proportional to apoptotic cell and cleaved caspase-3 densities and were inversely proportional to the density of cells showing Ki67 expression.
Conclusion: Diffusion-weighted imaging enabled measurement of early breast tumor response to TRA-8 treatment, prior to detectable tumor shrinkage, providing an effective mechanism to noninvasively monitor TRA-8 efficacy.
Supplemental material: http://radiology.rsnajnls.org/cgi/content/full/248/3/844/DC1.
RSNA, 2008
Figures






References
-
- Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997;277:818–821. - PubMed
-
- Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999;5:157–163. - PubMed
-
- Jo M, Kim TH, Seol DW, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med 2000;6:564–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

