Long-term survival of transplanted stem cells in immunocompetent mice with muscular dystrophy
- PMID: 18711004
- PMCID: PMC2527076
- DOI: 10.2353/ajpath.2008.080259
Long-term survival of transplanted stem cells in immunocompetent mice with muscular dystrophy
Abstract
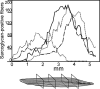
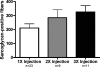
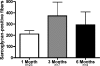
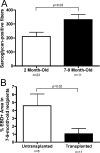
Satellite cells refer to resident stem cells in muscle that are activated in response to damage or disease for the regeneration and repair of muscle fibers. The use of stem cell transplantation to treat muscular diseases has been limited by impaired donor cell survival attributed to rejection and an unavailable stem cell niche. We isolated a population of adult muscle mononuclear cells (AMMCs) from normal, strain-matched muscle and transplanted these cells into delta-sarcoglycan-null dystrophic mice. Distinct from other transplant studies, the recipient mice were immunocompetent with an intact endogenous satellite cell pool. We found that AMMCs were 35 times more efficient at restoring sarcoglycan compared with cultured myoblasts. Unlike cultured myoblasts, AMMC-derived muscle fibers expressed sarcoglycan protein throughout their entire length, consistent with enhanced migratory ability. We examined the capacity of single injections of AMMCs to provide long-term benefit for muscular dystrophy and found persistent regeneration after 6 months, consistent with augmentation of the endogenous stem cell pool. Interestingly, AMMCs were more effectively engrafted into aged dystrophic mice for the regeneration of large clusters of sarcoglycan-positive muscle fibers, which were protected from damage, suggesting that the stem cell niche in older muscle remains permissive.
Figures




References
-
- Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–435. - PubMed
-
- Morgan JE, Watt DJ, Sloper JC, Partridge TA. Partial correction of an inherited biochemical defect of skeletal muscle by grafts of normal muscle precursor cells. J Neurol Sci. 1988;86:137–147. - PubMed
-
- Morgan JE. Practical aspects of myoblast implantation: investigations on two inherited myopathies in animals. Adv Exp Med Biol. 1990;280:86–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

