Receptor density balances signal stimulation and attenuation in membrane-assembled complexes of bacterial chemotaxis signaling proteins
- PMID: 18711126
- PMCID: PMC2527904
- DOI: 10.1073/pnas.0802868105
Receptor density balances signal stimulation and attenuation in membrane-assembled complexes of bacterial chemotaxis signaling proteins
Abstract
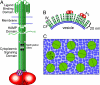
All cells possess transmembrane signaling systems that function in the environment of the lipid bilayer. In the Escherichia coli chemotaxis pathway, the binding of attractants to a two-dimensional array of receptors and signaling proteins simultaneously inhibits an associated kinase and stimulates receptor methylation--a slower process that restores kinase activity. These two opposing effects lead to robust adaptation toward stimuli through a physical mechanism that is not understood. Here, we provide evidence of a counterbalancing influence exerted by receptor density on kinase stimulation and receptor methylation. Receptor signaling complexes were reconstituted over a range of defined surface concentrations by using a template-directed assembly method, and the kinase and receptor methylation activities were measured. Kinase activity and methylation rates were both found to vary significantly with surface concentration--yet in opposite ways: samples prepared at high surface densities stimulated kinase activity more effectively than low-density samples, whereas lower surface densities produced greater methylation rates than higher densities. FRET experiments demonstrated that the cooperative change in kinase activity coincided with a change in the arrangement of the membrane-associated receptor domains. The counterbalancing influence of density on receptor methylation and kinase stimulation leads naturally to a model for signal regulation that is compatible with the known logic of the E. coli pathway. Density-dependent mechanisms are likely to be general and may operate when two or more membrane-related processes are influenced differently by the two-dimensional concentration of pathway elements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

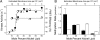

References
-
- Changeux JP, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. 2005;308:1424–1428. - PubMed
-
- Bray D, Duke T. Conformational spread: The propagation of allosteric states in large multiprotein complexes. Annu Rev Biophys Biomol Struct. 2004;33:53–73. - PubMed
-
- Maddock JR, Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993;259:1717–1723. - PubMed
-
- Kentner D, Sourjik V. Spatial organization of the bacterial chemotaxis system. Curr Opin Microbiol. 2006;9:619–624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

