Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes
- PMID: 18711143
- PMCID: PMC2527933
- DOI: 10.1073/pnas.0803153105
Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes
Erratum in
- Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):17206. DeGeorge, Brent Jr [corrected to DeGeorge, Brent R Jr]
Abstract
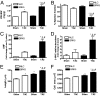
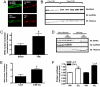
G protein-coupled receptor (GPCR) kinases (GRKs) are critical regulators of cellular signaling and function. In cardiomyocytes, GRK2 and GRK5 are two GRKs important for myocardial regulation, and both have been shown to be up-regulated in the dysfunctional heart. We report that increased levels and activity of GRK5 in failing myocardium may have unique significance due to its nuclear localization, a property not shared by GRK2. We find that transgenic mice with elevated cardiac GRK5 levels have exaggerated hypertrophy and early heart failure compared with control mice after pressure overload. This pathology is not present in cardiac GRK2-overexpressing mice or in mice with overexpression of a mutant GRK5 that is excluded from the nucleus. Nuclear accumulation of GRK5 is enhanced in myocytes after aortic banding in vivo and in vitro in myocytes after increased G alpha q activity, the trigger for pressure-overload hypertrophy. GRK5 enhances activation of MEF2 in concert with Gq signals, demonstrating that nuclear localized GRK5 regulates gene transcription via a pathway critically linked to myocardial hypertrophy. Mechanistically, we show that this is due to GRK5 acting, in a non-GPCR manner, as a class II histone deacetylase (HDAC) kinase because it can associate with and phosphorylate the myocyte enhancer factor-2 repressor, HDAC5. Moreover, significant HDAC activity can be found with GRK5 in the heart. Our data show that GRK5 is a nuclear HDAC kinase that plays a key role in maladaptive cardiac hypertrophy apparently independent of any action directly on GPCRs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



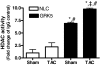
References
-
- Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. - PubMed
-
- Dzimiri N, Muiya P, Andres E, Al-Halees Z. Differential functional expression of human myocardial G protein receptor kinases in left ventricular cardiac diseases. Eur J Pharmacol. 2004;489:167–177. - PubMed
-
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. - PubMed
-
- Eckhart AD, et al. Hybrid transgenic mice reveal in vivo specificity of G protein-coupled receptor kinases in the heart. Circ Res. 2000;86:43–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

