Differential subcellular distribution and colocalization of the microsomal and soluble epoxide hydrolases in cultured neonatal rat brain cortical astrocytes
- PMID: 18711743
- PMCID: PMC3592782
- DOI: 10.1002/jnr.21827
Differential subcellular distribution and colocalization of the microsomal and soluble epoxide hydrolases in cultured neonatal rat brain cortical astrocytes
Abstract
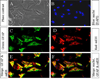
The microsomal epoxide hydrolase (mEH) and soluble epoxide hydrolase (sEH) enzymes exist in a variety of cells and tissues, including liver, kidney, and testis. However, very little is known about brain epoxide hydrolases. Here we report the expression, localization, and subcellular distribution of mEH and sEH in cultured neonatal rat cortical astrocytes by immunocytochemistry, subcellular fractionation, Western blotting, and radiometric enzyme assays. Our results showed a diffuse immunofluorescence pattern for mEH, which colocalized with the astroglial cytoskeletal marker glial fibrillary acidic protein (GFAP). The GFAP-positive cells also expressed sEH, which was localized mainly in the cytoplasm, especially in and around the nucleus. Western blot analyses revealed a distinct protein band with a molecular mass of approximately 50 kDa, the signal intensity of which increased about 1.5-fold in the microsomal fraction over the whole-cell lysate and other subcellular fractions. The polyclonal anti-human sEH rabbit serum recognized a protein band with a molecular mass similar to that of the affinity-purified sEH protein (approximately 62 kDa), the signal intensity of which increased over 1.7-fold in the 105,000g supernatant fraction over the cell lysate. Furthermore, the corresponding enzyme activities measured by using mEH- and sEH-selective substrates generally corroborated the immunocytochemical and Western blotting data. These results suggest that rat brain cortical astrocytes differentially coexpress mEH and sEH enzymes. The differential subcellular localization of mEH and sEH may play a role in the cerebrovascular functions that are known to be affected by brain-derived vasoactive epoxides.
2008 Wiley-Liss, Inc.
Figures


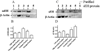
References
-
- Amruthesh SC, Boerschel MF, Mckinney JS, Willoughby KA, Ellis EF. Metabolism of Arachidonic Acid to Epoxyeicosatrienoic Acids, Hydroxyeicosatetraenoic Acids, and Prostaglandins in Cultured Rat Hippocampal Astrocytes. J.Neurochem. 1993;61:150–159. - PubMed
-
- Arand M, Cronin A, Adamska M, Oesch F. Epoxide Hydrolases: Structure, Function, Mechanism, and Assay. In. Methods in Enzymol. 2005;400:569–588. - PubMed
-
- Borhan B, Mebrahtu T, Nazarian S, Kurth MJ, Hammock BD. Improved radiolabeled substrates for soluble epoxide hydrolase. Anal Biochem. 1995;231:188–200. - PubMed
-
- Bradford MM. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41:163–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

