Mechanism of T cell tolerance induction by murine hepatic Kupffer cells
- PMID: 18712788
- PMCID: PMC2600585
- DOI: 10.1002/hep.22395
Mechanism of T cell tolerance induction by murine hepatic Kupffer cells
Abstract
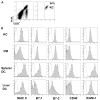
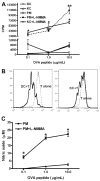
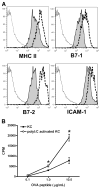
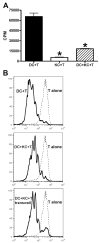
The liver is known to favor the induction of immunological tolerance rather than immunity. Although Kupffer cells (KC) have been indicated to play a role in liver tolerance to allografts and soluble antigens, the mechanisms involved remain unclear. We hypothesized that KCs could promote immune tolerance by acting as incompetent antigen-presenting cells (APC), as well as actively suppressing T cell activation induced by other potent APCs. The expression of antigen presentation-related molecules by KCs was phenotyped by flow cytometry. The abilities of KCs to act as APCs and to suppress T cell activation induced by splenic dendritic cells (DC) were examined by in vitro proliferation assays using CD4(+) OVA-TCR (ovalbumin T cell receptor) transgenic T cells. We found that, compared with DCs, KCs expressed significantly lower levels of major histocompatibility complex (MHC) II, B7-1, B7-2, and CD40. This result is consistent with our observation that KCs were not as potent as DCs in eliciting OVA-specific T cell proliferation. However, KCs isolated from polyinosinic:polycytidylic acid-treated mice expressed significantly higher levels of MHC II and costimulatory molecules than did naïve KCs and could stimulate stronger T cell responses. More importantly, we found that KCs could inhibit DC-induced OVA-specific T cell activation. Further investigation of the underlying mechanism revealed that prostaglandins produced by KCs played an important role. The results ruled out the possible involvement of interleukin-10, nitric oxide, 2,3-dioxygenase, and transforming growth factor beta in KC-mediated T cell suppression.
Conclusion: Our data indicate that KCs are a tolerogenic APC population within the liver. These findings suggest that KCs may play a critical role in regulating immune reactions within the liver and contributing to liver-mediated systemic immune tolerance. (HEPATOLOGY 2008.).
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures



References
-
- Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. - PubMed
-
- Gorczynski RM, Chan Z, Chung S, Cohen Z, Levy G, Sullivan B, et al. Prolongation of rat small bowel or renal allograft survival by pretransplant transfusion and/or by varying the route of allograft venous drainage. Transplantation. 1994;58:816–820. - PubMed
-
- Rao VK, Burris DE, Gruel SM, Sollinger HW, Burlingham WJ. Evidence that donor spleen cells administered through the portal vein prolong the survival of cardiac allografts in rats. Transplantation. 1988;45:1145–1146. - PubMed
-
- Cantor HM, Dumont AE. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature. 1967;215:744–745. - PubMed
-
- Chen Y, Ong CR, McKenna GJ, Mui AL, Smith RM, Chung SW. Induction of immune hyporesponsiveness after portal vein immunization with ovalbumin. Surgery. 2001;129:66–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
