A new cytogenetic mechanism for bacterial endosymbiont-induced parthenogenesis in Hymenoptera
- PMID: 18713719
- PMCID: PMC2605818
- DOI: 10.1098/rspb.2008.0792
A new cytogenetic mechanism for bacterial endosymbiont-induced parthenogenesis in Hymenoptera
Abstract
Vertically transmitted endosymbiotic bacteria, such as Wolbachia, Cardinium and Rickettsia, modify host reproduction in several ways to facilitate their own spread. One such modification results in parthenogenesis induction, where males, which are unable to transmit the bacteria, are not produced. In Hymenoptera, the mechanism of diploidization due to Wolbachia infection, known as gamete duplication, is a post-meiotic modification. During gamete duplication, the meiotic mechanism is normal, but in the first mitosis the anaphase is aborted. The two haploid sets of chromosomes do not separate and thus result in a single nucleus containing two identical sets of haploid chromosomes. Here, we outline an alternative cytogenetic mechanism for bacterial endosymbiont-induced parthenogenesis in Hymenoptera. During female gamete formation in Rickettsia-infected Neochrysocharis formosa (Westwood) parasitoids, meiotic cells undergo only a single equational division followed by the expulsion of a single polar body. This absence of meiotic recombination and reduction corresponds well with a non-segregation pattern in the offspring of heterozygous females. We conclude that diploidy in N. formosa is maintained through a functionally apomictic cloning mechanism that differs entirely from the mechanism induced by Wolbachia.
Figures
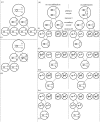


References
-
- Arakaki N, Kinjo K. Notes on the parasitoid fauna of the serpentine leafminer Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) in Okinawa, southern Japan. Appl. Entomol. Zool. 1998;33:577–581.
-
- Belshaw R, Quicke D.L.J. The cytogenetics of thelytoky in a predominantly asexual parasitoid wasp with covert sex. Genome. 2003;46:170–173. doi:10.1139/g02-112 - DOI - PubMed
-
- Bourtzis K, Miller T.A. CRC Press; Boca Raton, FL: 2003. Insect symbiosis. p. 347.
-
- Brownstein M.J, Carpten J.D, Smith J.R. Modulation of non-templated nucleotide addition by tag DNA polymerase: primer modifications that facilitate genotyping. Biotechniques. 1996;20:1004–1010. - PubMed
-
- Cook J.M. Sex determination in Hymenoptera: a review of models and evidence. Heredity. 1993;71:421–435. doi:10.1038/hdy.1993.157 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
