Small heat shock protein activity is regulated by variable oligomeric substructure
- PMID: 18713743
- PMCID: PMC2661405
- DOI: 10.1074/jbc.M804729200
Small heat shock protein activity is regulated by variable oligomeric substructure
Abstract
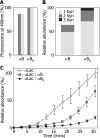
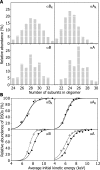
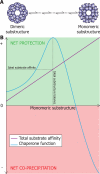
The alpha-crystallins are members of the small heat shock protein family of molecular chaperones that have evolved to minimize intracellular protein aggregation; however, they are also implicated in a number of protein deposition diseases. In this study, we employed novel mass spectrometry techniques to investigate the changes in quaternary structure associated with this switch from chaperone to adjuvant of aggregation. We replicated the oligomeric rearrangements observed for post-translationally modified alpha-crystallins, without altering the protein sequence, by refolding the alpha-crystallins in vitro. This refolding resulted in a loss of dimeric substructure concomitant with an augmentation of substrate affinity. We show that packaging of small heat shock proteins into dimeric units is used to control the level of chaperone function by regulating the exposure of hydrophobic surfaces. We propose that a bias toward monomeric substructure is responsible for the aberrant chaperone behavior associated with the alpha-crystallins in protein deposition diseases.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

